Page 223 - Basic Principles of Textile Coloration
P. 223
212 DYEING THEORY
20
Time of half dyeing/min 15
10
5
0 60
0 15 30 45
Number of passages of complete dye liquor
through fibre in 10 min
Key
Chlorazol Violet NS
Chlorazol Green GS
Chlorazol Sky Blue FFS
Durazol Helio BS
Durazol Fast Yellow 6 GS
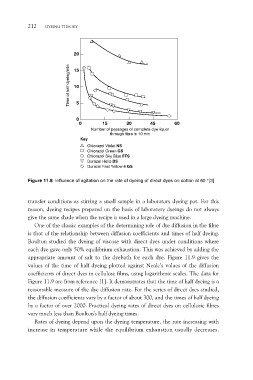
Figure 11.8 Influence of agitation on the rate of dyeing of direct dyes on cotton at 60 °[3]
transfer conditions as stirring a small sample in a laboratory dyeing pot. For this
reason, dyeing recipes prepared on the basis of laboratory dyeings do not always
give the same shade when the recipe is used in a large dyeing machine.
One of the classic examples of the determining role of dye diffusion in the fibre
is that of the relationship between diffusion coefficients and times of half dyeing.
Boulton studied the dyeing of viscose with direct dyes under conditions where
each dye gave only 50% equilibrium exhaustion. This was achieved by adding the
appropriate amount of salt to the dyebath for each dye. Figure 11.9 gives the
values of the time of half dyeing plotted against Neale’s values of the diffusion
coefficients of direct dyes in cellulose films, using logarithmic scales. The data for
Figure 11.9 are from reference [1]. It demonstrates that the time of half dyeing is a
reasonable measure of the dye diffusion rate. For the series of direct dyes studied,
the diffusion coefficients vary by a factor of about 300, and the times of half dyeing
by a factor of over 2000. Practical dyeing rates of direct dyes on cellulosic fibres
vary much less than Boulton’s half dyeing times.
Rates of dyeing depend upon the dyeing temperature, the rate increasing with
increase in temperature while the equilibrium exhaustion usually decreases.