Page 323 - Basic Principles of Textile Coloration
P. 323
312 DISPERSE DYES
SO3
CH3O OH HC OCH3
CH2 CH OH
(a) HO SO3 OH Glucose ring
CH2CO2H O CH CH OCH3
CH3O
OC O OCH3
CH3O CH OH
H HOCH2 CH OCH3
CH3 CH CH3 CH2 OH
CH2 CH CH SO3
(b) CH3 CH CH3 CH2 CH3 CH CH3
CH2 CH2 CH3 CH2
SO3 CH SO3
CH3
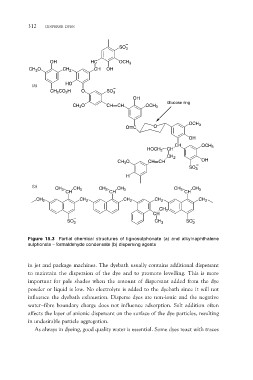
Figure 15.3 Partial chemical structures of lignosulphonate (a) and alkylnaphthalene
sulphonate – formaldehyde condensate (b) dispersing agents
in jet and package machines. The dyebath usually contains additional dispersant
to maintain the dispersion of the dye and to promote levelling. This is more
important for pale shades when the amount of dispersant added from the dye
powder or liquid is low. No electrolyte is added to the dyebath since it will not
influence the dyebath exhaustion. Disperse dyes are non-ionic and the negative
water–fibre boundary charge does not influence adsorption. Salt addition often
affects the layer of anionic dispersant on the surface of the dye particles, resulting
in undesirable particle aggregation.
As always in dyeing, good quality water is essential. Some dyes react with traces