Page 345 - Basic Principles of Textile Coloration
P. 345
334 REACTIVE DYES
Cl N NH Cl SO3 HN
NN ON NN
NH
N O NH N Cl
SO3
Cl
1
NH2SO2 SO3
N
NH N
N Cu N
N NH Cl
N NN
O3S SO2NH N Cl
2
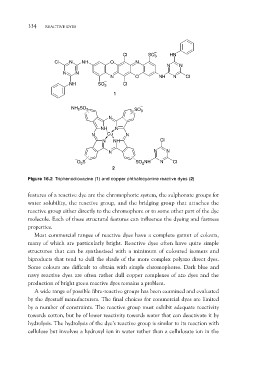
Figure 16.2 Triphenodioxazine (1) and copper phthalocyanine reactive dyes (2)
features of a reactive dye are the chromophoric system, the sulphonate groups for
water solubility, the reactive group, and the bridging group that attaches the
reactive group either directly to the chromophore or to some other part of the dye
molecule. Each of these structural features can influence the dyeing and fastness
properties.
Most commercial ranges of reactive dyes have a complete gamut of colours,
many of which are particularly bright. Reactive dyes often have quite simple
structures that can be synthesised with a minimum of coloured isomers and
biproducts that tend to dull the shade of the more complex polyazo direct dyes.
Some colours are difficult to obtain with simple chromophores. Dark blue and
navy reactive dyes are often rather dull copper complexes of azo dyes and the
production of bright green reactive dyes remains a problem.
A wide range of possible fibre-reactive groups has been examined and evaluated
by the dyestuff manufacturers. The final choices for commercial dyes are limited
by a number of constraints. The reactive group must exhibit adequate reactivity
towards cotton, but be of lower reactivity towards water that can deactivate it by
hydrolysis. The hydrolysis of the dye’s reactive group is similar to its reaction with
cellulose but involves a hydroxyl ion in water rather than a cellulosate ion in the