Page 347 - Basic Principles of Textile Coloration
P. 347
336 REACTIVE DYES
Cl Cl F
NN NN NN
Dye NH N Cl Dye NH N NHR Dye NH N NHR
Dichlorotriazine (DCT) Monochlorotriazine (MCT) Monofluorotriazine (MFT)
NHR CO2H Cl N Cl
NN Cl
Dye NH N N
N
Nicotinyltriazine (NT)
Dye NH N Cl Dye CO N Cl
Trichloropyrimidine (TCP) Dichloroquinoxaline (DCQ)
F Dye SO2 CH CH2
Cl Vinylsulphone (VS)
N
Dye NH N F
Difluorochloropyrimidine (DFCP)
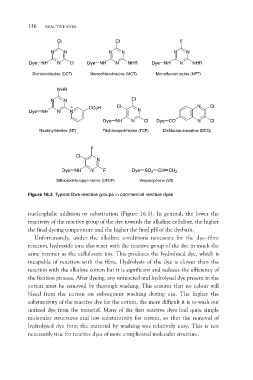
Figure 16.3 Typical fibre-reactive groups in commercial reactive dyes
nucleophilic addition or substitution (Figure 16.1). In general, the lower the
reactivity of the reactive group of the dye towards the alkaline cellulose, the higher
the final dyeing temperature and the higher the final pH of the dyebath.
Unfortunately, under the alkaline conditions necessary for the dye–fibre
reaction, hydroxide ions also react with the reactive group of the dye in much the
same manner as the cellulosate ion. This produces the hydrolysed dye, which is
incapable of reaction with the fibre. Hydrolysis of the dye is slower than the
reaction with the alkaline cotton but it is significant and reduces the efficiency of
the fixation process. After dyeing, any unreacted and hydrolysed dye present in the
cotton must be removed by thorough washing. This ensures that no colour will
bleed from the cotton on subsequent washing during use. The higher the
substantivity of the reactive dye for the cotton, the more difficult it is to wash out
unfixed dye from the material. Many of the first reactive dyes had quite simple
molecular structures and low substantivity for cotton, so that the removal of
hydrolysed dye from the material by washing was relatively easy. This is not
necessarily true for reactive dyes of more complicated molecular structure.