Page 348 - Basic Principles of Textile Coloration
P. 348
REACTIVE DYES FOR COTTON 337
16.2.3 Dye reactivity, application and storage
The reactive groups of the various types of reactive dye have different chemical
structures and show a wide range of reactivities. They were originally divided into
cold- and hot-dyeing types but many current ranges would be better called warm-
dyeing. The most reactive types, such as DCT reactive dyes (Figure 16.3), are
applied at lower temperatures (20–40 °C) and only require a weak alkali such as
NaHCO3 or Na2CO3 for fixation. The less reactive types, such as MCT dyes, need
higher temperatures (80–90 °C) and stronger alkalis such as Na2CO3 plus NaOH.
Many dyestuff manufacturers now market several ranges of reactive dyes for
cotton, each with its own particular recommended dyeing procedure. Table 16.1
gives some typical examples based on the type of reactive grouping.
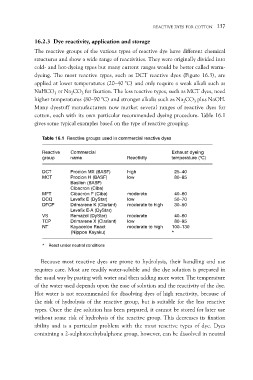
Table 16.1 Reactive groups used in commercial reactive dyes
Reactive Commercial Reactivity Exhaust dyeing
group name temperature (°C)
DCT Procion MX (BASF) high 25–40
MCT Procion H (BASF) low 80–85
Basilen (BASF)
MFT Cibacron (Ciba) moderate 40–60
DCQ Cibacron F (Ciba) low 50–70
DFCP Levafix E (DyStar) moderate to high 30–50
Drimarene K (Clariant)
VS Levafix E-A (DyStar) moderate 40–60
TCP Remazol (DyStar) low 80–95
NT Drimarene X (Clariant) moderate to high 100–130
Kayacelon React *
(Nippon Kayaku)
* React under neutral conditions
Because most reactive dyes are prone to hydrolysis, their handling and use
requires care. Most are readily water-soluble and the dye solution is prepared in
the usual way by pasting with water and then adding more water. The temperature
of the water used depends upon the ease of solution and the reactivity of the dye.
Hot water is not recommended for dissolving dyes of high reactivity, because of
the risk of hydrolysis of the reactive group, but is suitable for the less reactive
types. Once the dye solution has been prepared, it cannot be stored for later use
without some risk of hydrolysis of the reactive group. This decreases its fixation
ability and is a particular problem with the most reactive types of dye. Dyes
containing a 2-sulphatoethylsulphone group, however, can be dissolved in neutral