Page 653 - Cardiac Nursing
P. 653
CHAPTER 26 / Mechanical Circulatory Assist Devices 629
Percutaneous Centrifugal Pumps LVAD to the aorta (or into the pulmonary artery if it is in RVAD
Another percutaneously placed VAD is the left atrial to femoral configuration). Negative pressure is applied after ejection, caus-
artery VAD (e.g., Tandem Heart TM ). A venous catheter is placed ing the blood sac to fill. Backward flow is prevented by placement
in the left atrium via transseptal puncture from the right atrium. of inflow and outflow disk valves in the pump. The blood sac is
An arterial cannula is inserted in the iliac artery. The pump is a filled by means of a cannula placed in either the left atrium or
centrifugal model providing continuous flow. The device is used the LV. It can be controlled in one of three modes: (1) a fixed rate
for short-term stabilization, for hemodynamic support during that is asynchronous with the patient’s heart and delivers variable
PCIs, or as a bridge to recovery or surgical treatment. It requires stroke volumes, (2) triggering of the pump by the R wave of
anticoagulation (ideal activated clotting time of 250 to 350 the ECG (not practical for long-term support or in ambulatory
seconds). Studies have shown improvement in cardiac output (the patients), or (3) triggering of pump ejection by reaching full-fill
pump can move 4 to 5 L/min of blood), cardiac index, and other (also called fill-to-empty mode). 36 Infectious risk is increased in
metabolic parameters. Mortality rates were comparable between the paracorporeal device (where the pump is external). Conduits
IABP and VAD, but there were more complications (e.g., bleed- from the atrium or ventricle and the return conduits to the aorta
ing, acute limb ischemia) with VAD than with IABP. 32 are tunneled through the chest and connected to the external
Another centrifugal percutaneous VAD is the Biomedicus pump (Fig. 26-8). For longer-term support, the preference is to
(Medtronic, Inc., Minneapolis, MN) system. It is a centrifugal- cannulate the ventricle as larger flows can be obtained. Epithelial
kinetic energy pump that provides continuous flow via rotating cells grow into the Dacron-covered conduits and protect the pa-
cones that pull blood into the resulting vortex. It can be placed at tient from infection. Tissue growth acts as a seal from the surface
the bedside with cannulae inserted in the femoral vessels, thus not of the body.
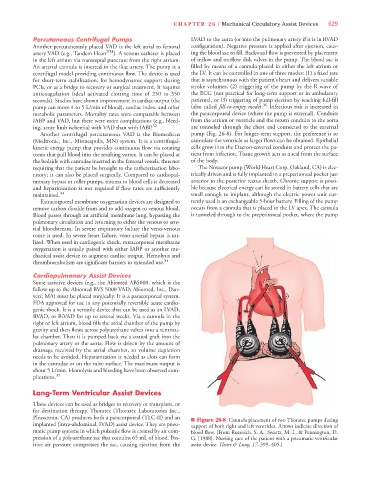
requiring that the patient be brought to the catheterization labo- The Novacor pump (World Heart Corp, Oakland, CA) is elec-
ratory; it can also be placed surgically. Compared to cardiopul- trically driven and is fully implanted in a preperitoneal pocket just
monary bypass or roller pumps, trauma to blood cells is decreased anterior to the posterior rectus sheath. Chronic support is possi-
and heparinization is not required if flow rates are sufficiently ble because electrical energy can be stored in battery cells that are
maintained. 33 small enough to implant, although the electric power unit cur-
Extracorporeal membrane oxygenation devices are designed to rently used is an exchangeable 5-hour battery. Filling of the pump
remove carbon dioxide from and to add oxygen to venous blood. occurs from a cannula that is placed in the LV apex. The cannula
Blood passes through an artificial membrane lung, bypassing the is tunneled through to the preperitoneal pocket, where the pump
pulmonary circulation and returning to either the venous or arte-
rial bloodstream. In severe respiratory failure the veno-venous
route is used. In severe heart failure, veno-arterial bypass is uti-
lized. When used in cardiogenic shock, extracorporeal membrane
oxygenation is usually paired with either IABP or another me-
chanical assist device to augment cardiac output. Hemolysis and
thromboembolism are significant barriers to extended use. 34
Cardiopulmonary Assist Devices
Some assistive devices (e.g., the Abiomed AB5000, which is the
follow-up to the Abiomed BVS 5000 VAD; Abiomed, Inc., Dan-
vers, MA) must be placed surgically. It is a paracorporeal system,
FDA approved for use in any potentially reversible acute cardio-
genic shock. It is a versatile device that can be used as an LVAD,
RVAD, or BiVAD for up to several weeks. Via a cannula in the
right or left atrium, blood fills the atrial chamber of the pump by
gravity and then flows across polyurethane valves into a ventricu-
lar chamber. Then it is pumped back via a coated graft into the
pulmonary artery or the aorta. Flow is driven by the amount of
drainage received by the atrial chamber, so volume depletion
needs to be avoided. Heparanization is needed as clots can form
in the cannulae or on the valve surface. The maximum output is
about 5 L/min. Hemolysis and bleeding have been observed com-
plications. 35
Long-Term Ventricular Assist Devices
These devices can be used as bridges to recovery or transplant, or
for destination therapy. Thoratec (Thoratec Laboratories Inc.,
Pleasanton, CA) produces both a paracorporeal (TLC-II) and an n Figure 26-8 Cannula placement of two Thoratec pumps during
implanted (intra-abdominal, IVAD) assist device. They are pneu- support of both right and left ventricles. Arrows indicate direction of
matic pump systems in which pulsatile flow is created by air com- blood flow. (From Ruzevich, S. A., Swartz, M. I., & Pennington, D.
pression of a polyurethane sac that contains 65 mL of blood. Pos- G. [1988]. Nursing care of the patient with a pneumatic ventricular
itive air pressure compresses the sac, causing ejection from the assist device. Heart & Lung, 17, 399–405.)