Page 1043 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1043
926 Part VII Hematologic Malignancies
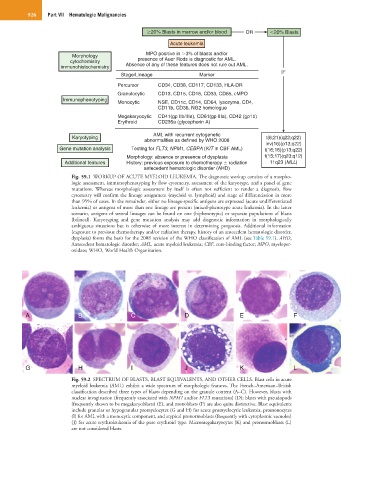
20% Blasts in marrow and/or blood OR 20% Blasts
Acute leukemia
Morphology MPO positive in 3% of blasts and/or
cytochemistry presence of Auer Rods is diagnostic for AML.
immunohistochemistry Absence of any of these features does not rule out AML.
IF
Stage/Lineage Marker
Percursor CD34, CD38, CD117, CD133, HLA-DR
Granulocytic CD13, CD15, CD16, CD33, CD65, cMPO
Immunophenotyping
Monocytic NSE, CD11c, CD14, CD64, lysozyme, CD4,
CD11b, CD36, NG2 homologue
Megakaryocytic CD41(gp IIb/IIIa), CD61(gp IIIa), CD42 (gp1b)
Erythroid CD235a (glycophorin A)
AML with recurrent cytogenetic
Karyotyping t(8;21)(q22;q22)
abnormalities as defined by WHO 2008
inv(16)(p13;q22)
Gene mutation analysis Testing for FLT3, NPM1, CEBPA (KIT in CBF AML) t(16;16)(p13;q22)
Morphology: absence or presence of dysplasia t(15;17)(q23;q12)
Additional features History: previous exposure to chemotherapy radiation 11q23 (MLL)
antecedent hematologic disorder (AHD)
Fig. 59.1 WORKUP OF ACUTE MYELOID LEUKEMIA. The diagnostic workup consists of a morpho-
logic assessment, immunophenotyping by flow cytometry, assessment of the karyotype, and a panel of gene
mutations. Whereas morphologic assessment by itself is often not sufficient to render a diagnosis, flow
cytometry will confirm the lineage assignment (myeloid vs. lymphoid) and stage of differentiation in more
than 95% of cases. In the remainder, either no lineage-specific antigens are expressed (acute undifferentiated
leukemia) or antigens of more than one lineage are present (mixed-phenotype acute leukemia). In the latter
scenario, antigens of several lineages can be found on one (biphenotypic) or separate populations of blasts
(bilineal). Karyotyping and gene mutation analysis may add diagnostic information in morphologically
ambiguous situations but is otherwise of more interest in determining prognosis. Additional information
(exposure to previous chemotherapy and/or radiation therapy, history of an antecedent hematologic disorder,
dysplasia) forms the basis for the 2008 revision of the WHO classification of AML (see Table 59.1). AHD,
Antecedent hematologic disorder; AML, acute myeloid leukemia; CBF, core-binding factor; MPO, myeloper-
oxidase; WHO, World Health Organization.
A B C D E F
G H I J K L
Fig. 59.2 SPECTRUM OF BLASTS, BLAST EQUIVALENTS, AND OTHER CELLS. Blast cells in acute
myeloid leukemia (AML) exhibit a wide spectrum of morphologic features. The French–American–British
classification described three types of blasts depending on the granule content (A–C). However, blasts with
nuclear invagination (frequently associated with NPM1 and/or FLT3 mutations) (D); blasts with pseudopods
(frequently shown to be megakaryoblasts) (E); and monoblasts (F) are also quite distinctive. Blast equivalents
include granular or hypogranular promyelocytes (G and H) for acute promyelocytic leukemia, promonocytes
(I) for AML with a monocytic component, and atypical pronormoblasts (frequently with cytoplasmic vacuoles)
(J) for acute erythroleukemia of the pure erythroid type. Micromegakaryocytes (K) and pronormoblasts (L)
are not considered blasts.