Page 1048 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1048
Chapter 59 Clinical Manifestations and Treatment of Acute Myeloid Leukemia 931
A A BB CC DD
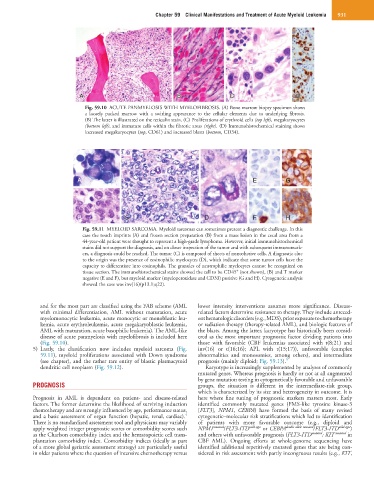
Fig. 59.10 ACUTE PANMYELOSIS WITH MYELOFIBROSIS. (A) Bone marrow biopsy specimen shows
a loosely packed marrow with a swirling appearance to the cellular elements due to underlying fibrosis.
(B) The latter is illustrated on the reticulin stain. (C) Proliferations of erythroid cells (top left), megakaryocytes
(bottom left), and immature cells within the fibrotic areas (right). (D) Immunohistochemical staining shows
increased megakaryocytes (top, CD61) and increased blasts (bottom, CD34).
A E G
B C C D F H
Fig. 59.11 MYELOID SARCOMA. Myeloid sarcomas can sometimes present a diagnostic challenge. In this
case the touch imprints (A) and frozen section preparation (B) from a mass lesion in the cecal area from a
44-year-old patient were thought to represent a high-grade lymphoma. However, initial immunohistochemical
stains did not support the diagnosis, and on closer inspection of the tumor and with subsequent immunomark-
ers, a diagnosis could be reached. The tumor (C) is composed of sheets of noncohesive cells. A diagnostic clue
to the origin was the presence of eosinophilic myelocytes (D), which indicate that some tumor cells have the
capacity to differentiate into eosinophils. The granules of neutrophilic myelocytes cannot be recognized on
+
tissue section. The immunohistochemical stains showed the cell to be CD45 (not shown), (B) and T marker
negative (E and F), but myeloid marker (myeloperoxidase and CD33) positive (G and H). Cytogenetic analysis
showed the case was inv(16)(p13.1;q22).
and for the most part are classified using the FAB scheme (AML lower intensity interventions assumes more significance. Disease-
with minimal differentiation, AML without maturation, acute related factors determine resistance to therapy. They include anteced-
myelomonocytic leukemia, acute monocytic or monoblastic leu- ent hematologic disorders (e.g., MDS), prior exposure to chemotherapy
kemia, acute erythroleukemia, acute megakaryoblastic leukemia, or radiation therapy (therapy-related AML), and biologic features of
AML with maturation, acute basophilic leukemia). The AML-like the blasts. Among the latter, karyotype has historically been consid-
disease of acute panmyelosis with myelofibrosis is included here ered as the most important prognostic factor dividing patients into
(Fig. 59.10). those with favorable (CBF leukemias associated with t(8;21) and
5) Lastly, the classification now includes myeloid sarcoma (Fig. inv(16) or t(16;16); APL with t(15;17)), unfavorable (complex
59.11), myeloid proliferations associated with Down syndrome abnormalities and monosomies, among others), and intermediate
(see chapter), and the rather rare entity of blastic plasmacytoid prognosis (mainly diploid; Fig. 59.13). 5
dendritic cell neoplasm (Fig. 59.12). Karyotype is increasingly supplemented by analyses of commonly
mutated genes. Whereas prognosis is hardly or not at all augmented
by gene mutation testing in cytogenetically favorable and unfavorable
PROGNOSIS groups, the situation is different in the intermediate-risk group,
which is characterized by its size and heterogeneity in outcome. It is
Prognosis in AML is dependent on patient- and disease-related here where fine tuning of prognostic markers matters most. Early
factors. The former determine the likelihood of surviving induction identified commonly mutated genes (FMS-like tyrosine kinase-3
chemotherapy and are strongly influenced by age, performance status, [FLT3], NPM1, CEBPA) have formed the basis of many revised
4
and a basic assessment of organ function (hepatic, renal, cardiac). cytogenetic–molecular risk stratifications which led to identification
There is no standardized assessment tool and physicians may variably of patients with more favorable outcome (e.g., diploid and
apply weighted integer prognostic scores or comorbidity scores such NPM1 mutated /FLT3-ITD wild-type or CEBPA double allele mutated /FLT3-ITD wild-type )
as the Charlson comorbidity index and the hematopoietic cell trans- and others with unfavorable prognosis (FLT3-ITD mutated ; KIT mutated in
plantation comorbidity index. Comorbidity indices (ideally as part CBF AML). Ongoing efforts at whole-genome sequencing have
of a more global geriatric assessment strategy) are particularly useful identified additional repetitively mutated genes that are being con-
in older patients where the question of intensive chemotherapy versus sidered in risk assessment with partly incongruous results (e.g., KIT,