Page 1047 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1047
930 Part VII Hematologic Malignancies
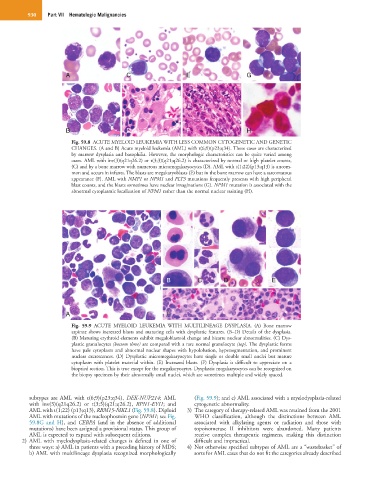
A C E G
B D F H
Fig. 59.8 ACUTE MYELOID LEUKEMIA WITH LESS COMMON CYTOGENETIC AND GENETIC
CHANGES. (A and B) Acute myeloid leukemia (AML) with t(6;9)(p23;q34). These cases are characterized
by marrow dysplasia and basophilia. However, the morphologic characteristics can be quite varied among
cases. AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2) is characterized by normal or high platelet counts,
(C) and by a bone marrow with numerous micromegakaryocytes (D). AML with t(1;22)(p13;q13) is uncom-
mon and occurs in infants. The blasts are megakaryoblasts (E) but in the bone marrow can have a sarcomatous
appearance (F). AML with NMP1 or NPM1 and FLT3 mutations frequently presents with high peripheral
blast counts, and the blasts sometimes have nuclear invaginations (G). NPM1 mutation is associated with the
abnormal cytoplasmic localization of NPM1 rather than the normal nuclear staining (H).
B C D E
A F F
Fig. 59.9 ACUTE MYELOID LEUKEMIA WITH MULTILINEAGE DYSPLASIA. (A) Bone marrow
aspirate shows increased blasts and maturing cells with dysplastic features. (B–D) Details of the dysplasia.
(B) Maturing erythroid elements exhibit megaloblastoid change and bizarre nuclear abnormalities. (C) Dys-
plastic granulocytes (bottom three) are compared with a rare normal granulocyte (top). The dysplastic forms
have pale cytoplasm and abnormal nuclear shapes with hypolobation, hypersegmentation, and prominent
nuclear excrescences. (D) Dysplastic micromegakaryocytes have single or double small nuclei but mature
cytoplasm with platelet material within. (E) Increased blasts. (F) Dysplasia is difficult to appreciate on a
biopsied section. This is true except for the megakaryocytes. Dysplastic megakaryocytes can be recognized on
the biopsy specimen by their abnormally small nuclei, which are sometimes multiple and widely spaced.
subtypes are AML with t(6;9)(p23;q34), DEK-NUP214; AML (Fig. 59.9); and c) AML associated with a myelodysplasia-related
with inv(3)(q21q26.2) or t(3;3)(q21;q26.2), RPN1-EVI1; and cytogenetic abnormality.
AML with t(1;22) (p13;q13), RBM15-MKL1 (Fig. 59.8). Diploid 3) The category of therapy-related AML was retained from the 2001
AML with mutations of the nucleophosmin gene (NPM1; see Fig. WHO classification, although the distinctions between AML
59.8G and H), and CEBPA (and in the absence of additional associated with alkylating agents or radiation and those with
mutations) have been assigned a provisional status. This group of topoisomerase II inhibitors were abandoned. Many patients
AML is expected to expand with subsequent editions. receive complex therapeutic regimens, making this distinction
2) AML with myelodysplasia-related changes is defined in one of difficult and impractical.
three ways: a) AML in patients with a preceding history of MDS; 4) Not otherwise specified subtypes of AML are a “wastebasket” of
b) AML with multilineage dysplasia recognized morphologically sorts for AML cases that do not fit the categories already described