Page 1118 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1118
984 Part VII Hematologic Malignancies
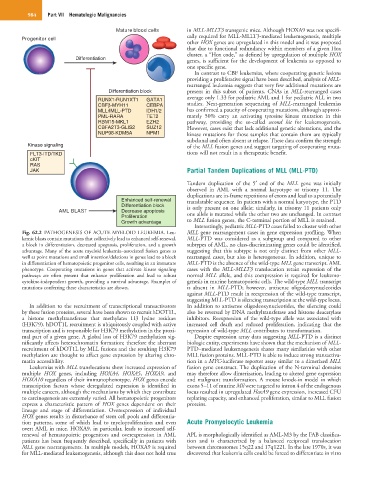
Mature blood cells in MLL-MLLT3 transgenic mice. Although HOXA9 was not specifi-
Progenitor cell cally required for MLL-MLLT3-mediated leukemogenesis, multiple
other HOX genes are upregulated in this model and it was proposed
that due to functional redundancy within members of a given Hox
cluster, a “Hox code,” as defined by upregulation of multiple HOX
Differentiation genes, is sufficient for the development of leukemia as opposed to
one specific gene.
In contrast to CBF leukemias, where cooperating genetic lesions
providing a proliferative signal have been described, analysis of MLL-
rearranged leukemia suggests that very few additional mutations are
Differentiation block present in this subset of patients. CNAs in MLL-rearranged cases
RUNX1-RUN1XT1 GATA1 average only 1.33 for pediatric AML and 1 for pediatric ALL in two
CBFβ-MYH11 CEBPA studies. Next-generation sequencing of MLL-rearranged leukemias
MLLrIMLL-PTD IDH1/2 has confirmed a paucity of cooperating mutations, although approxi-
PML-RARA TET2 mately 50% carry an activating tyrosine kinase mutation in this
RBM15-MKL1 EZH2 pathway, providing the so-called second hit for leukemogenesis.
CBFA2T3-GLIS2 SUZ12 However, cases exist that lack additional genetic alterations, and the
NUP98-KDM5A NPM1 kinase mutations for those samples that contain them are typically
subclonal and often absent at relapse. These data confirm the strength
Kinase signaling of the MLL fusion genes and suggest targeting of cooperating muta-
FLT3-ITD/TKD tions will not result in a therapeutic benefit.
cKIT
RAS
JAK Partial Tandem Duplications of MLL (MLL-PTD)
Tandem duplication of the 5′ end of the MLL gene was initially
observed in AML with a normal karyotype or trisomy 11. The
duplications are in-frame repetitions of exons and lead to a potentially
Enhanced self-renewal translatable sequence. In patients with a normal karyotype, the PTD
Differentiation block is only present on one allele; similarly, in trisomy 11 patients only
AML BLAST Decrease apoptosis
Proliferation one allele is mutated while the other two are unchanged. In contrast
Growth advantage to MLL fusion genes, the C-terminal portion of MLL is retained.
Interestingly, pediatric MLL-PTD cases failed to cluster with other
Fig. 62.2 PATHOGENESIS OF ACUTE MYELOID LEUKEMIA. Leu- MLL gene rearrangement cases in gene expression profiling. When
kemic blasts contain mutations that collectively lead to enhanced self-renewal, MLL-PTD was considered as a subgroup and compared to other
a block in differentiation, decreased apoptosis, proliferation, and a growth subtypes of AML, no class-discriminating genes could be identified,
advantage. Many of the acute myeloid leukemia–associated fusion genes as suggesting that this subtype is not only distinct from other MLL-
well as point mutations and small insertion/deletions in genes lead to a block rearranged cases, but also is heterogeneous. In addition, unique to
in differentiation of hematopoietic progenitor cells, resulting in an immature MLL-PTD is the absence of the wild-type MLL gene transcript. AML
phenotype. Cooperating mutations in genes that activate kinase signaling cases with the MLL-MLLT3 translocation retain expression of the
pathways are often present that enhance proliferation and lead to robust normal MLL allele, and this coexpression is required for leukemo-
cytokine-independent growth, providing a survival advantage. Examples of genesis in murine hematopoietic cells. The wild-type MLL transcript
mutations conferring these characteristics are shown. is absent in MLL-PTD; however, antisense oligodeoxynucleotides
against MLL-PTD result in reexpression of the wild-type transcript,
suggesting MLL-PTD is silencing transcription at the wild-type locus.
In addition to the recruitment of transcriptional transactivators In addition to antisense oligodeoxynucleotides, the silencing could
by these fusion proteins, several have been shown to recruit hDOT1L, also be reversed by DNA methyltransferase and histone deacetylase
a histone methyltransferase that methylates H3 lysine residues inhibitors. Reexpression of the wild-type allele was associated with
(H3K79). hDOT1L recruitment is ubiquitously coupled with active increased cell death and reduced proliferation, indicating that the
transcription and is responsible for H3K79 methylation in the proxi- repression of wild-type MLL contributes to transformation.
mal part of a given gene. A global loss of H3K79 methylation sig- Despite expression array data suggesting MLL-PTD is a distinct
nificantly affects heterochromatin formation; therefore the aberrant biologic entity, experiments have shown that the mechanism of MLL-
recruitment of hDOT1L by MLL fusions and the resulting H3K79 PTD–mediated leukemogenesis shares many similarities with other
methylation are thought to affect gene expression by altering chro- MLL fusion proteins. MLL-PTD is able to induce strong transactiva-
matin accessibility. tion in a MYC-luciferase reporter assay similar to a dimerized MLL
Leukemias with MLL translocations show increased expression of fusion gene construct. The duplication of the N-terminal domains
multiple HOX genes, including HOXA4, HOXA5, HOXA9, and may therefore allow dimerization, leading to altered gene expression
HOXA10 regardless of their immunophenotype. HOX genes encode and malignant transformation. A mouse knock-in model in which
transcription factors whose deregulated expression is identified in exons 5–11 of murine Mll were targeted to intron 4 of the endogenous
multiple cancers, although the mechanisms by which they contribute locus resulted in upregulated HoxA9 gene expression, increased CFU
to carcinogenesis are extremely varied. All hematopoietic progenitors replating capacity, and enhanced proliferation, similar to MLL fusion
express a characteristic pattern of HOX genes dependent on their proteins.
lineage and stage of differentiation. Overexpression of individual
HOX genes results in disturbance of stem cell pools and differentia-
tion patterns, some of which lead to myeloproliferation and even Acute Promyelocytic Leukemia
overt AML in mice. HOXA9, in particular, leads to increased self-
renewal of hematopoietic progenitors and overexpression in AML APL is morphologically identified as AML-M3 by the FAB classifica-
patients has been frequently described, specifically in patients with tion and is characterized by a balanced reciprocal translocation
MLL gene rearrangements. In multiple models, HOXA9 is required between chromosomes 15q22 and 17q1221. In the late 1970s, it was
for MLL-mediated leukemogenesis, although this does not hold true discovered that leukemia cells could be forced to differentiate in vitro