Page 1196 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1196
1044 Part VII Hematologic Malignancies
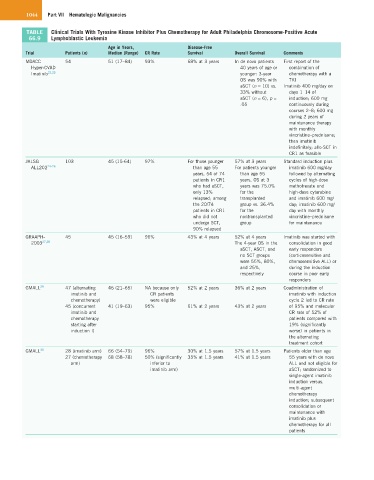
TABLE Clinical Trials With Tyrosine Kinase Inhibitor Plus Chemotherapy for Adult Philadelphia Chromosome-Positive Acute
66.9 Lymphoblastic Leukemia
Age in Years, Disease-Free
Trial Patients (n) Median (Range) CR Rate Survival Overall Survival Comments
MDACC 54 51 (17–84) 93% 68% at 3 years In de novo patients First report of the
Hyper-CVAD 40 years of age or combination of
Imatinib 22,23 younger: 3-year chemotherapy with a
OS was 90% with TKI
aSCT (n = 10) vs. Imatinib 400 mg/day on
33% without days 1–14 of
aSCT (n = 6), p = induction; 600 mg
.05 continuously during
courses 2–8; 600 mg
during 2 years of
maintenance therapy
with monthly
vincristine–prednisone;
then imatinib
indefinitely; allo-SCT in
CR1 as feasible
JALSG 103 45 (15-64) 97% For those younger 57% at 3 years Standard induction plus
ALL202 24–26 than age 55 For patients younger imatinib 600 mg/day
years, 54 of 74 than age 55 followed by alternating
patients in CR1 years, OS at 3 cycles of high-dose
who had aSCT, years was 75.0% methotrexate and
only 13% for the high-dose cytarabine
relapsed; among transplanted and imatinib 600 mg/
the 20/74 group vs. 36.4% day; imatinib 600 mg/
patients in CR1 for the day with monthly
who did not nontransplanted vincristine–prednisone
undergo SCT, group for maintenance
90% relapsed
GRAAPH- 45 45 (16–59) 96% 43% at 4 years 52% at 4 years Imatinib was started with
2003 27,28 The 4-year OS in the consolidation in good
aSCT, ASCT, and early responders
no SCT groups (corticosensitive and
were 55%, 80%, chemosensitive ALL) or
and 25%, during the induction
respectively course in poor early
responders
GMALL 29 47 (alternating 46 (21–65) NA because only 52% at 2 years 36% at 2 years Coadministration of
imatinib and CR patients imatinib with induction
chemotherapy) were eligible cycle 2 led to CR rate
45 (concurrent 41 (19–63) 95% 61% at 2 years 43% at 2 years of 95% and molecular
imatinib and CR rate of 52% of
chemotherapy patients compared with
starting after 19% (significantly
induction I) worse) in patients in
the alternating
treatment cohort
GMALL 30 28 (imatinib arm) 66 (54–79) 96% 30% at 1.5 years 57% at 1.5 years Patients older than age
27 (chemotherapy 68 (58–78) 50% (significantly 35% at 1.5 years 41% at 1.5 years 55 years with de novo
arm) inferior to ALL and not eligible for
imatinib arm) aSCT; randomized to
single-agent imatinib
induction versus.
multi-agent
chemotherapy
induction; subsequent
consolidation or
maintenance with
imatinib plus
chemotherapy for all
patients