Page 1360 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1360
1206 Part VII Hematologic Malignancies
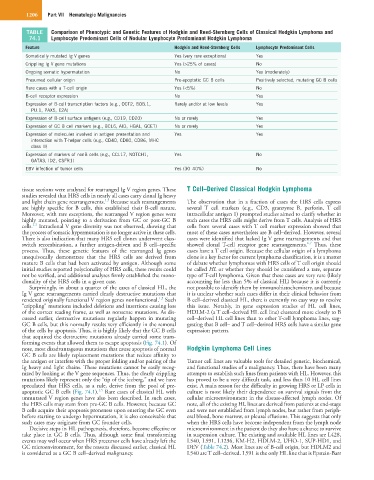
TABLE Comparison of Phenotypic and Genetic Features of Hodgkin and Reed-Sternberg Cells of Classical Hodgkin Lymphoma and
74.1 Lymphocyte Predominant Cells of Nodular Lymphocyte Predominant Hodgkin Lymphoma
Feature Hodgkin and Reed-Sternberg Cells Lymphocyte Predominant Cells
Somatically mutated Ig V genes Yes (very rare exceptions) Yes
Crippling Ig V gene mutations Yes (>25% of cases) No
Ongoing somatic hypermutation No Yes (moderately)
Presumed cellular origin Pre-apoptotic GC B cells Positively selected, mutating GC B cells
Rare cases with a T-cell origin Yes (<5%) No
B-cell receptor expression No Yes
Expression of B-cell transcription factors (e.g., OCT2, BOB.1, Rarely and/or at low levels Yes
PU.1, PAX5, E2A)
Expression of B-cell surface antigens (e.g., CD19, CD20) No or rarely Yes
Expression of GC B cell markers (e.g., BCL6, AID, HGAL, GCET) No or rarely Yes
Expression of molecules involved in antigen presentation and Yes Yes
interaction with T-helper cells (e.g., CD40, CD80, CD86, MHC
class II)
Expression of markers of nonB cells (e.g., CCL17, NOTCH1, Yes No
GATA3, ID2, CSFR1)
EBV infection of tumor cells Yes (30–40%) No
tissue sections were analyzed for rearranged Ig V region genes. These T Cell–Derived Classical Hodgkin Lymphoma
studies revealed that HRS cells in nearly all cases carry clonal Ig heavy
1,5
and light chain gene rearrangements. Because such rearrangements The observation that in a fraction of cases the HRS cells express
are highly specific for B cells, this established their B-cell nature. several T cell markers (e.g., CD3, granzyme B, perforin, T cell
Moreover, with rare exceptions, the rearranged V region genes were intracellular antigen 1) prompted studies aimed to clarify whether in
highly mutated, pointing to a derivation from GC or post-GC B such cases the HRS cells might derive from T cells. Analysis of HRS
1,5
cells. Intraclonal V gene diversity was not observed, showing that cells from several cases with T cell marker expression showed that
the process of somatic hypermutation is no longer active in these cells. most of these cases nevertheless are B cell–derived. However, several
There is also indication that many HRS cell clones underwent class- cases were identified that lacked Ig V gene rearrangements and that
6,7
switch recombination, a further antigen-driven and B cell–specific showed clonal T-cell receptor gene rearrangements. Thus, these
process. Thus, these genetic features of the rearranged Ig genes cases have a T cell origin. Because the cellular origin of a lymphoma
unequivocally demonstrate that the HRS cells are derived from clone is a key factor for current lymphoma classification, it is a matter
mature B cells that had been activated by antigen. Although some of debate whether lymphomas with HRS cells of T cell origin should
initial studies reported polyclonality of HRS cells, these results could be called HL or whether they should be considered a rare, separate
not be verified, and additional analyses firmly established the mono- type of T-cell lymphoma. Given that these cases are very rare (likely
clonality of the HRS cells in a given case. accounting for less than 5% of classical HL) because it is currently
Surprisingly, in about a quarter of the cases of classical HL, the not possible to identify them by immunohistochemistry, and because
Ig V gene rearrangements carried clearly destructive mutations that it is unclear whether such cases differ in their clinical behavior from
1,5
rendered originally functional V region genes nonfunctional. Such B cell–derived classical HL, there is currently no easy way to resolve
“crippling” mutations included deletions and insertions causing loss this issue. Notably, in gene expression studies of HL cell lines,
of the correct reading frame, as well as nonsense mutations. As dis- HDLM-2 (a T cell–derived HL cell line) clustered more closely to B
cussed earlier, destructive mutations regularly happen in mutating cell–derived HL cell lines than to other T-cell lymphoma lines, sug-
GC B cells, but this normally results very efficiently in the removal gesting that B cell– and T cell–derived HRS cells have a similar gene
of the cells by apoptosis. Thus, it is highly likely that the GC B cells expression pattern.
that acquired the destructive mutations already carried some trans-
forming events that allowed them to escape apoptosis (Fig. 74.1). Of
note, most disadvantageous mutations that cause apoptosis of normal Hodgkin Lymphoma Cell Lines
GC B cells are likely replacement mutations that reduce affinity to
the antigen or interfere with the proper folding and/or pairing of the Tumor cell lines are valuable tools for detailed genetic, biochemical,
Ig heavy and light chains. These mutations cannot be easily recog- and functional studies of a malignancy. Thus, there have been many
nized by looking at the V gene sequences. Thus, the clearly crippling attempts to establish such lines from patients with HL. However, this
mutations likely represent only the “tip of the iceberg,” and we have has proved to be a very difficult task, and less than 10 HL cell lines
speculated that HRS cells, as a rule, derive from the pool of pre- exist. A main reason for the difficulty in growing HRS or LP cells in
1,5
apoptotic GC B cells (Fig. 74.1). Rare cases of classical HL with culture is most likely their dependence on survival signals from the
unmutated V region genes have also been described. In such cases, cellular microenvironment in the disease-affected lymph nodes. Of
the HRS cells may stem from pre-GC B cells. However, because GC note, all of the existing HL lines are derived from patients at end-stage
B cells acquire their apoptosis proneness upon entering the GC even and were not established from lymph nodes, but rather from periph-
before starting to undergo hypermutation, it is also conceivable that eral blood, bone marrow, or pleural effusions. This suggests that only
such cases may originate from GC founder cells. when the HRS cells have become independent from the lymph node
Decisive steps in HL pathogenesis, therefore, become effective or microenvironment in the patient do they also have a chance to survive
take place in GC B cells. Thus, although some final transforming in suspension culture. The existing and available HL lines are L428,
events may well occur when HRS precursor cells have already left the L540, L591, L1236, KM-H2, HDLM-2, UHO-1, SUP-HD1, and
GC microenvironment, for the reasons discussed earlier, classical HL DEV (Table 74.2). Most lines are of B-cell origin, but HDLM2 and
is considered as a GC B cell–derived malignancy. L540 are T cell–derived. L591 is the only HL line that is Epstein-Barr