Page 1426 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1426
1272 Part VII Hematologic Malignancies
1.0 + + + + + + + + + + + + + 1.0 CR + Hb and Pit higher
Proportion of patients disease free 0.7 + Cladribine + + + + + + + + + + + + + + + Pentostatin + Proportion of patients remaining relapse free 0.8 PR + Hb and Pit higher
0.9
+
0.9
0.8
(n = 88)
+
+
+
0.7
++
+ + +
+
+
+
0.6
CR + Hb and/or Pit lower
0.6
+ + + + + +
+ + + + + + +
+
+
(n = 90)
0.5
0.5
0.4
0.4
0.3
(n = 15)
0.2
Relapse or HCL death
0.2
0.1
0.1
0.0 + Censored 0.3 PR + Hb and/or Pit lower (n = 19)
P <0.0001
0 2 4 6 8 10 12 14 16 18 20 0.0
A Time in years 0 2 4 6 8 10 12 14 16 18 20
A Years from first purine analogue treatment
120
CR PR 1.0 Second to sixth line:
100 0.9 purine analogue + rituximab (n = 12)
Percentage of patients 80 Proportion of patients remaining relapse free 0.7 Second/third line:
0.8
CR (n = 61)
60
0.6
0.5
First line:
40
CR (n = 186)
20 0.4 First line:
0.3
0 0.2 Second/third line: PR (n = 37)
1 2 3 0.1 PR (n = 29) P <0.0001
B Course of purine analogue therapy 0.0
0 2 4 6 8 10 12 14 16 18 20
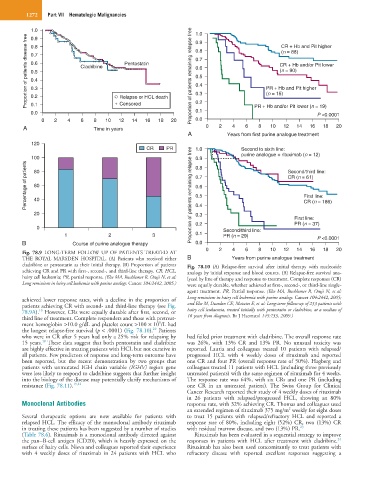
Fig. 78.9 LONG-TERM FOLLOW UP OF PATIENTS TREATED AT
THE ROYAL MARSDEN HOSPITAL. (A) Patients who received either B Years from purine analogue treatment
cladribine or pentostatin as their initial therapy. (B) Proportion of patients Fig. 78.10 (A) Relapse-free survival after initial therapy with nucleoside
achieving CR and PR with first-, second-, and third-line therapy. CR, HCL, analogs by initial response and blood counts. (B) Relapse-free survival ana-
hairy cell leukemia; PR, partial response. (Else MA, Ruchlemer R, Osuji N, et al: lyzed by line of therapy and response to treatment. Complete responses (CR)
Long remissions in hairy cell leukemia with purine analogs. Cancer 104:2442, 2005.) were equally durable, whether achieved at first-, second-, or third-line single-
agent treatment. PR, Partial response. (Else MA, Ruchlemer R, Osuji N, et al:
achieved lower response rates, with a decline in the proportion of Long remissions in hairy cell leukemia with purine analogs. Cancer 104:2442, 2005;
patients achieving CR with second- and third-line therapy (see Fig. and Else M, Dearden CE, Matutes E, et al: Long-term follow-up of 233 patients with
10
78.9A). However, CRs were equally durable after first, second, or hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of
third line of treatment. Complete responders and those with pretreat- 16 years from diagnosis. Br J Haematol 145:733, 2009.)
9
ment hemoglobin >10.0 g/dL and platelet count >100 × 10 /L had
10
the longest relapse-free survival (p < .0001) (Fig. 78.10). Patients
who were in CR after 5 years had only a 25% risk for relapsing by had failed prior treatment with cladribine. The overall response rate
10
15 years. These data suggest that both pentostatin and cladribine was 26%, with 13% CR and 13% PR. No unusual toxicity was
are highly effective in treating patients with HCL but not curative in reported. Lauria and colleagues treated 10 patients with relapsed/
all patients. Few predictors of response and long-term outcome have progressed HCL with 4 weekly doses of rituximab and reported
been reported, but the recent demonstration by two groups that one CR and four PR (overall response rate of 50%). Hagberg and
patients with unmutated IGH chain variable (IGHV) region gene colleagues treated 11 patients with HCL (including three previously
were less likely to respond to cladribine suggests that further insight untreated patients) with the same regimen of rituximab for 4 weeks.
into the biology of the disease may potentially clarify mechanisms of The response rate was 64%, with six CRs and one PR (including
resistance (Fig. 78.11). 19,22 one CR in an untreated patient). The Swiss Group for Clinical
Cancer Research reported their study of 4 weekly doses of rituximab
in 26 patients with relapsed/progressed HCL, showing an 80%
Monoclonal Antibodies response rate, with 32% achieving CR. Thomas and colleagues used
2
an extended regimen of rituximab 375 mg/m weekly for eight doses
Several therapeutic options are now available for patients with to treat 15 patients with relapsed/refractory HCL and reported a
relapsed HCL. The efficacy of the monoclonal antibody rituximab response rate of 80%, including eight (52%) CR, two (13%) CR
in treating these patients has been suggested by a number of studies with residual marrow disease, and two (13%) PR. 23
(Table 78.6). Rituximab is a monoclonal antibody directed against Rituximab has been evaluated in a sequential strategy to improve
24
the pan−B-cell antigen (CD20), which is heavily expressed on the responses in patients with HCL after treatment with cladribine.
surface of hairy cells. Nieva and colleagues reported their experience Rituximab has also been used concomitantly to treat patients with
with 4 weekly doses of rituximab in 24 patients with HCL who refractory disease with reported excellent responses suggesting a