Page 1424 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1424
1270 Part VII Hematologic Malignancies
TABLE Selected Published Reports of Cladribine Therapy for TABLE Alternative Dose and Schedules of Cladribine Therapy
78.3 Hairy Cell Leukemia 78.4 Reported in the Literature
Evaluable Route of
Reference Patients (n) CR (%) PR (%) OR (%) Study Dosing Administration Responses
Intravenous Daily Administration (Continuous or Pulsed) Saven 15 0.1 mg/kg/day × Continuous IV CR: 91%; PR:
Saven 15 349 91 7 98 7 days infusion 7%
2
Goodman 16 207 95 5 100 Juliusson 14 3.4 mg/m /day × Subcutaneous CR: 75% after
7 days injection 1 cycle, 85%
Tallman 50 80 18 98
after 2 cycles
Chadha 17 85 79 21 100
Robak 13 0.12 mg/kg/day × 2-hour IV CR: 76%; PR:
Hoffman 49 76 24 100 5 days bolus 19%
Seymore 18 46 78 11 89 Robak 13 0.12 mg/kg/week × 2 hour IV CR: 72%; PR:
Dearden 45 84 16 100 6 weeks bolus 19%
Juliusson 16 75 0 75 Chacko 0.15 mg/kg/week × 3 hour IV CR: 100%
Zinzani a 21 81 19 100 6 weeks infusion
Jehn 44 98 2 100 Lauria 12 0.15 mg/kg/week × 2 hour IV CR: 76%; PR:
6 weeks bolus 24%
Robakb, 13 132 76 19 95
Intravenous Weekly Administration Von Rohr 0.14 mg/kg/day × Subcutaneous CR: 76%; PR:
Lauria 30 73 27 100 5 days injection 21%
CR, Complete response; IV, intravenous; PR, partial response.
Zinzani a 16 81 19 100
Robak b,13 57 72 19 91
Subcutaneous Administration 1.0
Juliusson 14 73 81 13 94 0.9
Forconi 19 58 72 19 91 CR + Hb and/or
a Reports from the same publication. 0.8 Plt higher (n = 88)
b Numbers reported for the two arms of a randomized study. 0.7
CR, Complete response; OR, overall response; PR, partial response. 0.6 CR + Hb and/or
Plt lower (n = 90)
analogs in treating lymphoid neoplasms can be traced back to the Proportion of patients remaining relapse free 0.5
0.4
observation that children with the deficiency of the enzyme adenine PR + Hb and
deaminase (ADA) developed severe combined immunodeficiency. 0.3 Plt higher (n =15)
The accumulation of the triphosphorylated form of deoxyadenosine 0.2
(dependent on the action of the enzyme deoxycitidine kinase [DCK]) PR + Hb and/or
is thought to be responsible for the lack of lymphocyte development. 0.1 Plt lower (n =19) P < 0.0001
Similarly, after treatment with purine nucleoside analogs, the accu- 0.0
mulation of deoxyadenosine triphosphate results in DNA strand 0 2 4 6 8 10 12 14 16 18 20
breaks, inhibition of DNA repair, and apoptosis preferentially in
lymphoid cells, rich in DCK and low in 5′ nucleotidase (the enzyme Years from first purine analogue treatment
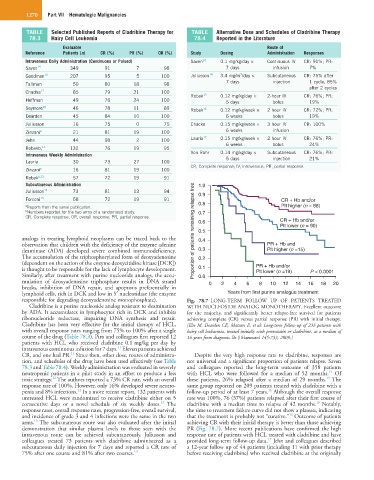
responsible for degrading deoxyadenosine monophosphate). Fig. 78.7 LONG-TERM FOLLOW UP OF PATIENTS TREATED
Cladribine is a purine nucleoside analog resistant to deamination WITH NUCLEOSIDE ANALOG MONOTHERAPY. Excellent outcome
by ADA. It accumulates in lymphocytes rich in DCK and inhibits for the majority, and significantly better relapse-free survival for patients
ribonucleotide reductase, impairing DNA synthesis and repair. achieving complete (CR) versus partial response (PR) with initial therapy.
Cladribine has been very effective for the initial therapy of HCL, (Else M, Dearden CE, Matutes E, et al: Long-term follow-up of 233 patients with
with overall response rates ranging from 75% to 100% after a single hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of
course of the drug (Table 78.3). Piro and colleagues first reported 12 16 years from diagnosis. Br J Haematol 145:733, 2009.)
patients with HCL who received cladribine 0.1 mg/kg per day by
11
intravenous continuous infusion for 7 days. Eleven patients achieved
11
CR, and one had PR. Since then, other dose, routes of administra- Despite the very high response rate to cladribine, responses are
tion, and schedules of the drug have been used effectively (see Table not universal and a significant proportion of patients relapse. Saven
78.3 and Table 78.4). Weekly administration was evaluated in severely and colleagues reported the long-term outcome of 358 patients
15
neutropenic patients in a pilot study in an effort to produce a less with HCL who were followed for a median of 52 months. Of
12
15
toxic strategy. The authors reported a 73% CR rate, with an overall these patients, 26% relapsed after a median of 29 months. The
response rate of 100%. However, only 16% developed severe neutro- same group reported on 209 patients treated with cladribine with a
12
16
penia and 8% infections. In a more recent report, 132 patients with follow-up period of at least 7 years. Although the overall response
untreated HCL were randomized to receive cladribine either on 5 rate was 100%, 76 (37%) patients relapsed after their first course of
16
13
consecutive days or a novel schedule of six weekly doses. The cladribine with a median time to relapse of 42 months. Notably,
response rates, overall response rates, progression-free, overall survival, the time to treatment failure curve did not show a plateau, indicating
16
and incidence of grade 3 and 4 infections were the same in the two that the treatment is probably not “curative.” Outcome of patients
13
arms. The subcutaneous route was also evaluated after the initial achieving CR with their initial therapy is better than those achieving
demonstration that similar plasma levels to those seen with the PR (Fig. 78.7). More recent publications have confirmed the high
intravenous route can be achieved subcutaneously. Juliusson and response rate of patients with HCL treated with cladribine and have
17
colleagues treated 73 patients with cladribine administered as a provided long-term follow-up data. Jehn and colleagues described
subcutaneous daily injection for 7 days and reported a CR rate of a 12-year follow up of 44 patients (including 11 with prior therapy
75% after one course and 81% after two courses. 14 before receiving cladribine) who received cladribine at the originally