Page 1427 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1427
Chapter 78 Hairy Cell Leukemia 1273
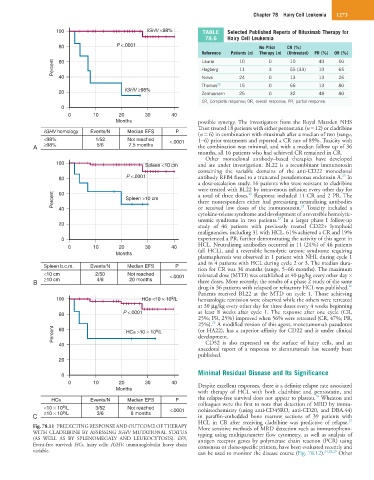
100 IGHV <98% TABLE Selected Published Reports of Rituximab Therapy for
78.6 Hairy Cell Leukemia
80 P <.0001 No Prior CR (%)
Reference Patients (n) Therapy (n) (Untreated) PR (%) OR (%)
Percent 60 Lauria 10 0 3 10 40 50
55 (33)
65
10
11
Hagberg
40 Nieva 24 0 13 13 26
Thomas 23 15 0 66 13 80
20 IGHV ≥98% Zenhausern 25 0 32 48 80
CR, Complete response; OR, overall response; PR, partial response.
0
0 10 20 30 40
Months possible synergy. The investigators from the Royal Marsden NHS
Trust treated 18 patients with either pentostatin (n = 12) or cladribine
IGHV homology Events/N Median EFS P
(n = 6) in combination with rituximab after a median of two (range,
<98% 1/52 Not reached <.0001 1−6) prior treatments and reported a CR rate of 89%. Toxicity with
A ≥98% 5/6 7.5 months the combination was minimal, and with a median follow up of 36
months, all 16 patients who had achieved CR remained in CR.
Other monoclonal antibody−based therapies have developed
100 Spleen <10 cm and are under investigation. BL22 is a recombinant immunotoxin
containing the variable domains of the anti-CD22 monoclonal
25
80 P <.0001 antibody RFB4 fused to a truncated pseudomonas endotoxin A. In
a dose-escalation study, 16 patients who were resistant to cladribine
were treated with BL22 by intravenous infusion every other day for
60
Percent Spleen >10 cm a total of three doses. Response included 11 CR and 2 PR. The
25
three nonresponders either had preexisting neutralizing antibodies
25
or received low doses of the immunotoxin. Toxicity included a
40
cytokine-release syndrome and development of a reversible hemolytic-
25
uremic syndrome in two patients. In a larger phase I follow-up
20 study of 46 patients with previously treated CD22+ lymphoid
malignancies, including 31 with HCL, 61% achieved a CR and 19%
0 experienced a PR, further demonstrating the activity of this agent in
0 10 20 30 40 HCL. Neutralizing antibodies occurred in 11 (24%) of 46 patients
Months (all HCL), and a reversible hemolytic uremic syndrome requiring
plasmapheresis was observed in 1 patient with NHL during cycle 1
and in 4 patients with HCL during cycle 2 or 3. The median dura-
Spleen b.c.m. Events/N Median EFS P tion for CR was 36 months (range, 5−66 months). The maximum
<10 cm 2/50 Not reached <.0001 tolerated dose (MTD) was established at 40 µg/kg every other day ×
B ≥10 cm 4/8 20 months three doses. More recently, the results of a phase 2 study of the same
26
drug in 36 patients with relapsed or refractory HCL was published.
Patients received BL22 at the MTD on cycle 1. Those achieving
9
100 HCs <10 × 10 /L hematologic remission were observed while the others were retreated
at 30 µg/kg every other day for three doses every 4 weeks beginning
80 P <.0001 at least 8 weeks after cycle 1. The response after one cycle (CR,
25%; PR, 25%) improved when 56% were retreated (CR, 47%; PR,
26
25%). A modified version of this agent, moxetumomab pasudotox
Percent 60 HCs >10 × 10 /L (or HA22), has a superior affinity for CD22 and is under clinical
9
development.
40 CD52 is also expressed on the surface of hairy cells, and an
anecdotal report of a response to alemtuzumab has recently been
published.
20
Minimal Residual Disease and Its Significance
0
0 10 20 30 40 Despite excellent responses, there is a definite relapse rate associated
Months
with therapy of HCL with both cladribine and pentostatin, and
10
HCs Events/N Median EFS P the relapse-free survival does not appear to plateau. Wheaton and
<10 × 10 /L 3/52 Not reached <.0001 colleagues were the first to note that detection of MRD by immu-
9
nohistochemistry (using anti-CD45RO, anti-CD20, and DBA.44)
9
C ≥10 × 10 /L 3/6 8 months in paraffin-embedded bone marrow sections of 39 patients with
27
HCL in CR after receiving cladribine was predictive of relapse.
Fig. 78.11 PREDICTING RESPONSE AND OUTCOME OF THERAPY More sensitive methods of MRD detection such as immunopheno-
WITH CLADRIBINE BY ASSESSING IGHV MUTATIONAL STATUS typing using multiparameter flow cytometry, as well as analysis of
(AS WELL AS BY SPLENOMEGALY AND LEUKOCYTOSIS). EFS, antigen receptor genes by polymerase chain reaction (PCR) using
Event-free survival; HCs, hairy cells; IGHV, immunoglobulin heavy chain consensus or clone-specific primers, have been evaluated recently and
variable. can be used to monitor the disease course (Fig. 78.12). 24,28,29 Other