Page 1425 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1425
Chapter 78 Hairy Cell Leukemia 1271
reported dose and schedule. The CR rate was 98%, and with a median 79%, with an overall response rate of 96%. With a median follow up
follow up of 8.5 years (range 0.1−12.2), 17 patients had relapsed. of 63.5 months, 34 (15%) of 220 responding patients had relapsed.
Eight of nine patients retreated with cladribine responded again. The estimated 5- and 10-year disease-free survival was 88% and
The overall survival at 12 years was 79%. Zinzani and colleagues 69%, respectively, and the estimated 5-year overall survival was 89%.
reported the long-term outcome of 37 patients treated with one of There are no prospective trials comparing the efficacy and durabil-
two regimens of cladribine. Twenty-one patients received cladribine ity of response between cladribine and pentostatin. Dearden and
by a 2-hour infusion for 5 days, whereas 16 patients were treated with colleagues examined the outcome of the patients with the two agents
a once-weekly schedule for 5 weeks. A CR rate of 81% with an overall at their institution and reported that 82% of 165 patients treated
response rate of 100% was reported, with no difference between with pentostatin achieved a CR compared with 84% of 45 patients
the two schedules. After a median follow up of 122 months (range treated with cladribine. Relapse rates were 24% with pentostatin and
54−156), the overall relapse rate was about 30% for both groups. The 29% with cladribine after median follow up of 71 and 45 months,
projected 13-year overall and relapse-free survival rates were 96% and respectively. These relapse rates suggest a longer remission duration
52%, respectively. Investigators at Northwestern University treated with pentostatin. However, with further follow up, there appears to
86 consecutive patients with cladribine and reported a CR rate of be no difference between the two agents with regard to disease-free
17
79%, as well as a PR rate of 21%. The progression-free survival survival (Fig. 78.9A). Further follow-up data of these cohorts of
10
after 12 years was 54%. After a median follow up of 9.7 years (range patients have been reported. With a median follow up of 16 years,
17
0.3−13.8), 31 patients (36%) relapsed. Of these, 23 were treated there was no significant difference in the outcome of the patients
with a second course of cladribine; 12 (52%) achieved CR, and 7 treated with the two drugs. After relapse or nonresponse, patients
17
(30%) achieved PR. The overall survival after 12 years was 87%. could be successfully retreated with pentostatin or cladribine but
The authors suggested that the lower CR rate in this study was
caused by their more stringent criteria for response, which included
a requirement for resolution of splenomegaly and lymphadenopathy 100%
by computed tomography scan as criteria for CR. 17
Similar excellent responses have been achieved using pentostatin
(Table 78.5). Overall CR rates of 44% to 89% have been reported
with pentostatin administered intravenously at a dose of 2−4 mg/ 80%
2
m every 2 weeks. Spiers and colleagues were the first to report that
a nucleoside analog (pentostatin) was capable of producing CRs in
patients with HCL. The activity of pentostatin in HCL was con- 60%
firmed in a number of larger studies by several investigators. Grever
and colleagues conducted a large, randomized clinical trial compar- 10-year
ing pentostatin with IFN-α in patients with previously untreated 40% At risk Events estimate
20
HCL. Patients were randomized to receive either IFN-α 3 million 117 31 67%
units subcutaneously three times per week or pentostatin 4 mg/ 56 16 69%
20
2
m intravenously every 2 weeks. Patients who did not respond to Initial induction
initial treatment were crossed over. Confirmed complete and overall 20% Crossover
response rates were reported for 76% and 79% of patients treated
with pentostatin, respectively, as compared with 11% and 38% of
20
those treated with IFN-α. Response rates were significantly higher 0%
(p < .0001), and relapse-free survival was significantly longer with 0 2 4 6 8 10 12
20
pentostatin than with interferon (p < .0001). Furthermore, patients A
who were initially assigned to receive interferon were frequently Years after complete response
crossed over to pentostatin therapy, achieving a CR rate of 66%.
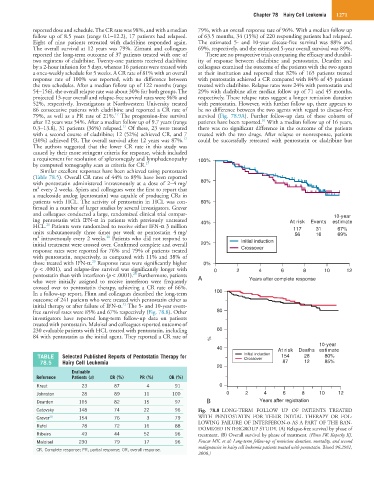
In a follow-up report, Flinn and colleagues described the long-term 100
outcome of 241 patients who were treated with pentostatin either as
21
initial therapy or after failure of IFN-α. The 5- and 10-year event-
free survival rates were 85% and 67% respectively (Fig. 78.8). Other 80
investigators have reported long-term follow-up data on patients
treated with pentostatin. Maloisel and colleagues reported outcome of
230 evaluable patients with HCL treated with pentostatin, including 60
84 with pentostatin as the initial agent. They reported a CR rate of
%
10-year
40 At risk Deaths estimate
TABLE Selected Published Reports of Pentostatin Therapy for Initial induction 154 28 80%
Crossover
78.5 Hairy Cell Leukemia 87 12 85%
20
Evaluable
Reference Patients (n) CR (%) PR (%) OR (%)
Kraut 23 87 4 91 0
Johnston 28 89 11 100 0 2 4 6 8 10 12
Dearden 165 82 15 97 B Years after registration
Catovsky 148 74 22 96 Fig. 78.8 LONG-TERM FOLLOW UP OF PATIENTS TREATED
Grever 20 154 76 3 79 WITH PENTOSTATIN FOR THEIR INITIAL THERAPY OR FOL-
LOWING FAILURE OF INTERFERON-α AS A PART OF THE RAN-
Rafel 78 72 16 88
DOMIZED INTERGROUP STUDY. (A) Relapse-free survival by phase of
Ribeiro 49 44 52 96 treatment. (B) Overall survival by phase of treatment. (Flinn IW, Kopecky KJ,
Maloisel 230 79 17 96 Foucar MK, et al: Long-term follow-up of remission duration, mortality, and second
malignancies in hairy cell leukemia patients treated with pentostatin. Blood 96:2981,
CR, Complete response; PR, partial response; OR, overall response.
2000.)