Page 1589 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1589
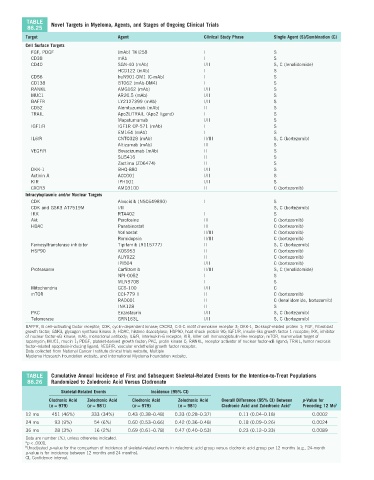
TABLE Novel Targets in Myeloma, Agents, and Stages of Ongoing Clinical Trials
86.25
Target Agent Clinical Study Phase Single Agent (S)/Combination (C)
Cell Surface Targets
FGF, PDGF (mAb) TKI258 I S
CD38 mAb I S
CD40 SGN-40 (mAb) I/II S, C (lenalidomide)
HCD122 (mAb) I S
CD56 huN901-DM1 (C-mAb) I S
CD138 BT062 (mAb-DM4) I S
RANKL AMG162 (mAb) I/II S
MUC1 AR20.5 (mAb) I/II S
BAFFR LY2127399 (mAb) I/II S
CD52 Alemtuzumab (mAb) II S
TRAIL Apo2L/TRAIL (Apo2 ligand) I S
Mapatumumab I/II S
IGF1/R IGF1R CP-571 (mAb) I S
EM164 (mAb) I S
IL6/R CNTO328 (mAb) II/III S, C (bortezomib)
Altizumab (mAb) III S
VEGF/R Bevacizumab (mAb) II S
SU5416 II S
Zactima (ZD6474) II S
DKK-1 BHQ-880 I/II S
Activin A ACC001 I/II S
KIR IPH101 I/II S
CXCR3 AMD3100 II C (bortezomib)
Intracytoplasmic and/or Nuclear Targets
CDK Alvocidib (NSC649890) I S
CDK and GSK3 AT7519M I/II S, C (bortezomib)
IKK RTA402 I S
Akt Perofosine III C (bortezomib)
HDAC Panabinostat III C (bortezomib)
Vorinostat II/III C (bortezomib)
Romidopsin II/III C (bortezomib)
Farnesyltransferase inhibitor Tipifarnib (R115777) II S, C (bortezomib)
HSP90 KOS953 II C (bortezomib)
AUY922 II C (bortezomib)
IPI504 I/II C (bortezomib)
Proteasome Carfilzomib II/III S, C (lenalidomide)
NPI-0052 I S
MLN9708 I S
Mitochondria GCS-100 I/II C
mTOR CCI-779 II II C (bortezomib)
RAD001 II C (lenalidomide, bortezomib)
INK128 II S
PKC Enzastaurin I/II S, C (bortezomib)
Telomerase GRN163L I/II S, C (bortezomib)
BAFFR, B cell–activating factor receptor; CDK, cyclin-dependent kinase; CXCR3, C-X-C motif chemokine receptor 3; DKK-1, Dickkopf-related protein 1; FGF, Fibroblast
growth factor; GSK3, glycogen synthase kinase 3; HDAC, histone deacetylase; HSP90, heat shock protein 90; IGF1/R, insulin-like growth factor 1 receptor; IKK, inhibitor
of nuclear factor-κB kinase; mAb, monoclonal antibody; IL6/R, interleukin-6 receptor; KIR, killer cell immunoglobulin-like receptor; mTOR, mammalian target of
rapamycin; MUC1, mucin 1; PDGF, platelet-derived growth factor; PKC, protin kinase C; RANKL, receptor activator of nuclear factor-κB ligand; TRAIL, tumor necrosis
factor–related apoptosis-inducing ligand; VEGF/R, vascular endothelial growth factor receptor.
Data collected from National Cancer Institute clinical trials website, Multiple
Myeloma Research Foundation website, and International Myeloma Foundation website.
TABLE Cumulative Annual Incidence of First and Subsequent Skeletal-Related Events for the Intention-to-Treat Populations
86.26 Randomized to Zoledronic Acid Versus Clodronate
Skeletal-Related Events Incidence (95% CI)
Clodronic Acid Zoledronic Acid Clodronic Acid Zoledronic Acid Overall Difference (95% CI) Between p-Value for
(n = 979) (n = 981) (n = 979) (n = 981) Clodronic Acid and Zoledronic Acid a Preceding 12 Mo b
12 mo 451 (46%) 333 (34%) 0.43 (0.38–0.48) 0.33 (0.28–0.37) 0.11 (0.04–0.18) 0.0002
24 mo 93 (9%) 54 (6%) 0.60 (0.53–0.66) 0.42 (0.36–0.48) 0.18 (0.09–0.26) 0.0024
36 mo 28 (3%) 16 (2%) 0.69 (0.61–0.78) 0.47 (0.40–0.53) 0.23 (0.12–0.33) 0.0089
Data are number (%), unless otherwise indicated.
a p < .0001.
b Unadjusted p-value for the comparison of incidence of skeletal-related events in zoledronic acid group versus clodronic acid group per 12 months (e.g., 24-month
p-value is for incidence between 12 months and 24 months).
CI, Confidence interval.