Page 1587 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1587
1414 Part VII Hematologic Malignancies
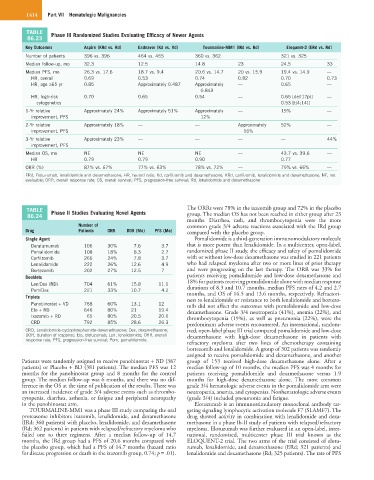
TABLE Phase III Randomized Studies Evaluating Efficacy of Newer Agents
86.23
Key Outcomes Aspire (KRd vs. Rd) Endeavor (Kd vs. Vd) Tourmaline-MM1 (IRd vs. Rd) Eloquent-2 (ERd vs. Rd)
Number of patients 396 vs. 396 464 vs. 465 360 vs. 362 321 vs. 325
Median follow-up, mo 32.3 12.5 14.8 23 24.5 33
Median PFS, mo 26.3 vs. 17.6 18.7 vs. 9.4 20.6 vs. 14.7 20 vs. 15.9 19.4 vs. 14.9 —
HR, overall 0.69 0.53 0.74 0.82 0.70 0.73
HR, age ≥65 yr 0.85 Approximately 0.487 Approximately — 0.65 —
0.843
HR, high-risk 0.70 0.65 0.54 — 0.65 [del(17p)] —
cytogenetics 0.53 [t(4;14)]
1-Yr relative Approximately 24% Approximately 51% Approximately — 19% —
improvement, PFS 12%
2-Yr relative Approximately 18% — — Approximately 52% —
improvement, PFS 16%
3-Yr relative Approximately 23% — — — — 44%
improvement, PFS
Median OS, mo NE NE NE — 43.7 vs. 39.6 —
HR 0.79 0.79 0.90 — 0.77 —
ORR (%) 87% vs. 67% 77% vs. 63% 78% vs. 72% — 79% vs. 66% —
ERd, Elotuzumab, lenalidomide and dexamethasone; HR, hazard ratio; Kd, carfilzomib and dexamethasone; KRd, carfilzomib, lenalidomide and dexamethasone; NE, not
evaluable; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; Rd, lenalidomide and dexamethasone.
TABLE Phase II Studies Evaluating Novel Agents The ORRs were 78% in the ixazomib group and 72% in the placebo
86.24 group. The median OS has not been reached in either group after 23
months. Diarrhea, rash, and thrombocytopenia were the more
Number of common grade 3/4 adverse reactions associated with the IRd group
Drug Patients ORR DOR (Mo) PFS (Mo) compared with the placebo group.
Single Agent Pomalidomide is a third-generation immunomodulatory molecule
Daratumumab 106 30% 7.6 3.7 that is more potent than lenalidomide. In a multicenter, open-label,
Pomalidomide 108 18% 8.3 2.7 randomized phase II study, the efficacy and safety of pomalidomide
Carfilzomib 266 24% 7.8 3.7 with or without low-dose dexamethasone was studied in 221 patients
Lenalidomide 222 26% 12.6 4.9 who had relapsed myeloma after two or more lines of prior therapy
Bortezomib 202 27% 12.5 7 and were progressing on the last therapy. The ORR was 33% for
Doublets patients receiving pomalidomide and low-dose dexamethasone and
Len/Dex (RD) 704 61% 15.8 11.1 18% for patients receiving pomalidomide alone with median response
durations of 8.3 and 10.7 months, median PFS rates of 4.2 and 2.7
Pom/Dex 221 33% 10.7 4.2 months, and OS of 16.5 and 13.6 months, respectively. Refractori-
Triplets ness to lenalidomide or resistance to both lenalidomide and bortezo-
Panobinostat + VD 768 60% 13.1 12 mib did not affect the outcomes with pomalidomide and low-dose
Elo + RD 646 80% 21 19.4 dexamethasone. Grade 3/4 neutropenia (41%), anemia (22%), and
Ixazomib + RD 65 80% 20.5 20.6 thrombocytopenia (19%), as well as pneumonia (22%), were the
CRD 792 85% 28.6 26.3 predominant adverse events encountered. An international, random-
CRD, Lenalidomide-cyclophosphamide-dexamethasone; Dex, dexamethasone; ized, open-label phase III trial compared pomalidomide and low-dose
DOR, duration of response; Elo, elotuzumab; Len, lenalidomide; ORR, overall dexamethasone with high-dose dexamethasone in patients with
response rate; PFS, progression-free survival; Pom, pomalidomide.
refractory myeloma after two lines of chemotherapy containing
bortezomib and lenalidomide. A group of 302 patients was randomly
assigned to receive pomalidomide and dexamethasone, and another
Patients were randomly assigned to receive panobinostat + BD (387 group of 153 received high-dose dexamethasone alone. After a
patients) or Placebo + BD (381 patients). The median PFS was 12 median follow-up of 10 months, the median PFS was 4 months for
months for the panobinostat group and 8 months for the control patients receiving pomalidomide and dexamethasone versus 1.9
group. The median follow-up was 6 months, and there was no dif- months for high-dose dexamethasone alone. The most common
ference in the OS at the time of publication of the results. There was grade 3/4 hematologic adverse events in the pomalidomide arm were
an increased incidence of grade 3/4 adverse events such as thrombo- neutropenia, anemia, and cytopenias. Nonhematologic adverse events
cytopenia, diarrhea, asthenia, or fatigue and peripheral neuropathy (grade 3/4) included pneumonia and fatigue.
in the panobinostat arm. Elotuzumab is an immunostimulatory monoclonal antibody tar-
TOURMALINE-MM1 was a phase III study comparing the oral geting signaling lymphocytic activation molecule F7 (SLAMF7). The
proteasome inhibitors ixazomib, lenalidomide, and dexamethasone drug showed activity in combination with lenalidomide and dexa-
(IRd; 360 patients) with placebo, lenalidomide, and dexamethasone methasone in a phase Ib-II study of patients with relapsed/refractory
(Rd; 362 patients) in patients with relapsed/refractory myeloma who myeloma. Elotuzumab was further evaluated in an open-label, inter-
failed one to three regimens. After a median follow-up of 14.7 national, randomized, multicenter phase III trial known as the
months, the IRd group had a PFS of 20.6 months compared with ELOQUENT-2 trial. The two arms of the trial consisted of elotu-
the placebo group, which had a PFS of 14.7 months (hazard ratio zumab, lenalidomide, and dexamethasone (ERd; 321 patients) and
for disease progression or death in the ixazomib group, 0.74; p = .01). lenalidomide and dexamethasone (Rd; 325 patients). The rate of PFS