Page 1590 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1590
Chapter 86 Plasma Cell Neoplasms 1417
Clochonic acid + Censored
40 Zoledronic acid 35% 100 = .0107
2
90
Patients with skeletal- related events (%) 20 HR-0.74 (95% Cl, 0.62-0.87); 27% Overall survival (% patients) 70 CLO (n = 682)
HR = 0.82 (0.6% Cl, 0.70, 0.06)
80
30
60
50
40
30
10
10
0 P = .0004 20 0 ZOL (n = 668)
0 6 21 18 24 30 36 42 0 1 2 3 4 5
A Time from randomization (mo) D Survival since initial randomization (yr)
Number at risk
Clochonic acid 979 629 465 337 256 173 112 74 ZOL 668 544 447 292 165 64
Zoledronic acid 0.5 981 663 506 390 284 201 138 97 CLO 100 662 534 + Censored 143 53
437
271
Cumulative incidence function, skeletal-related events (%) 0.4 HR-0.77 (95% Cl, HR, 0.53 (95% Cl, Overall survival (% patients) 90 CLO (n = 276)
2
= .4690
HR = 1.10 (95% Cl, 0.85, 1.60)
80
0.33-0.84)
70
log-rank P = .0068
60
0.3
50
40
0.2
30
20
0.65-0.92)
0.1
ZOL (n = 302)
10
log-rank P = .0038
0
0 6 121824303642485460
Survival since initial randomization (yr)
Time from randomization 0 6 121824303642485460 0 0 1 2 3 4 5
Time from randomization
B (mo) C (mo) E
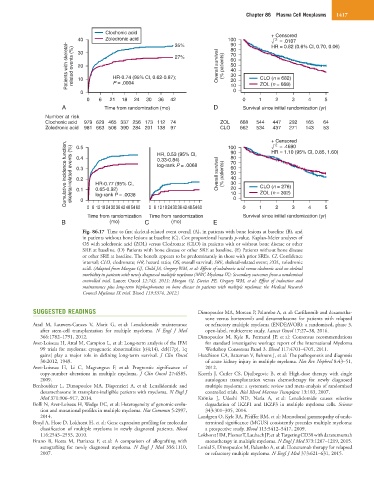
Fig. 86.17 Time to first skeletal-related event overall (A), in patients with bone lesions at baseline (B), and
in patients without bone lesions at baseline (C). Cox proportional hazards p-value. Kaplan-Meier analyses of
OS with zoledronic acid (ZOL) versus Clodronate (CLO) in patients with or without bone disease or other
SRE at baseline. (D) Patients with bone disease or other SRE at baseline. (E) Patients without bone disease
or other SRE at baseline. The benefit appears to be predominantly in those with prior SREs. CI, Confidence
interval; CLO, clodronate; HR, hazard ratio; OS, overall survival; SRE, skeletal-related event; ZOL, zoledronic
acid. (Adapted from Morgan GJ, Child JA, Gregory WM, et al: Effects of zoledronic acid versus clodronic acid on skeletal
morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): Secondary outcomes from a randomised
controlled trial. Lancet Oncol 12:743, 2011; Morgan GJ, Davies FE, Gregory WM, et al: Effect of induction and
maintenance plus long-term bisphosphonates on bone disease in patients with multiple myeloma: the Medical Research
Council Myeloma IX trial. Blood 119:5374, 2012.)
SUGGESTED READINGS Dimopoulos MA, Moreau P, Palumbo A, et al: Carfilzomib and dexametha-
sone versus bortezomib and dexamethasone for patients with relapsed
Attal M, Lauwers-Cances V, Marit G, et al: Lenalidomide maintenance or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3,
after stem-cell transplantation for multiple myeloma. N Engl J Med open-label, multicentre study. Lancet Oncol 17:27–38, 2016.
366:1782–1791, 2012. Dimopoulos M, Kyle R, Fermand JP, et al: Consensus recommendations
Avet-Loiseau H, Attal M, Campion L, et al: Long-term analysis of the IFM for standard investigative workup: report of the International Myeloma
99 trials for myeloma: cytogenetic abnormalities [t(4;14), del(17p), 1q Workshop Consensus Panel 3. Blood 117:4701–4705, 2011.
gains] play a major role in defining long-term survival. J Clin Oncol Hutchison CA, Batuman V, Behrens J, et al: The pathogenesis and diagnosis
30:2012, 1949. of acute kidney injury in multiple myeloma. Nat Rev Nephrol 8:43–51,
Avet-Loiseau H, Li C, Magrangeas F, et al: Prognostic significance of 2012.
copy-number alterations in multiple myeloma. J Clin Oncol 27:4585, Koreth J, Cutler CS, Djulbegovic B, et al: High-dose therapy with single
2009. autologous transplantation versus chemotherapy for newly diagnosed
Benboubker L, Dimopoulos MA, Dispenzieri A, et al: Lenalidomide and multiple myeloma: a systematic review and meta-analysis of randomized
dexamethasone in transplant-ineligible patients with myeloma. N Engl J controlled trials. Biol Blood Marrow Transplant 13:183, 2007.
Med 371:906–917, 2014. Krönke J, Udeshi ND, Narla A, et al: Lenalidomide causes selective
Bolli N, Avet-Loiseau H, Wedge DC, et al: Heterogeneity of genomic evolu- degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science
tion and mutational profiles in multiple myeloma. Nat Commun 5:2997, 343:301–305, 2014.
2014. Landgren O, Kyle RA, Pfeiffer RM, et al: Monoclonal gammopathy of unde-
Broyl A, Hose D, Lokhorst H, et al: Gene expression profiling for molecular termined significance (MGUS) consistently precedes multiple myeloma:
classification of multiple myeloma in newly diagnosed patients. Blood a prospective study. Blood 113:5412–5417, 2009.
116:2543–2553, 2010. Lokhorst HM, Plesner T, Laubach JP, et al: Targeting CD38 with daratumumab
Bruno B, Rotta M, Patriarca F, et al: A comparison of allografting with monotherapy in multiple myeloma. N Engl J Med 373:1207–1219, 2015.
autografting for newly diagnosed myeloma. N Engl J Med 356:1110, Lonial S, Dimopoulos M, Palumbo A, et al: Elotuzumab therapy for relapsed
2007. or refractory multiple myeloma. N Engl J Med 373:621–631, 2015.