Page 1614 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1614
Chapter 88 Immunoglobulin Light Chain Amyloidosis (Primary Amyloidosis) 1435
and be placed on corticosteroids without appropriate screening. The with the specific inclusion of Doppler studies that accurately measure
rarest physical finding in amyloidosis is periarticular infiltration of the restricted filling that occurs with amyloid infiltration (stiff heart
the synovium in the upper extremities, leading to the “shoulder pad” syndrome). It may also include magnetic resonance imaging of the
sign. This causes a continuous, chronic, low-grade pain caused by the heart, which can show distinct endomyocardial enhancement follow-
periarticular infiltration. Diagnosis in this setting requires arthrocen- ing gadolinium injection, as well as myocardial nulling, which is
tesis and the demonstration of Congo red–positive deposits in the specific for light-chain amyloidosis.
synovial fluid. A patient with unexplained hepatomegaly who has a monoclonal
protein can often avoid a liver biopsy, which rarely is associated with
bleeding and occasionally with hepatic rupture. Fat aspiration is
LABORATORY MANIFESTATIONS positive in over 75% of patients with hepatic amyloidosis.
In a patient with peripheral neuropathy who is found to have a
If the symptoms of amyloidosis are so vague as not to be helpful and monoclonal protein, consideration of amyloidosis in the differential
the physical findings are specific but not sensitive, when should a diagnosis can prevent interventions such as plasma exchange or
clinician suspect amyloidosis and aggressively pursue this rare disor- intravenous immunoglobulin infusions, which are ineffective in
der? Amyloidosis should be considered in any patient who presents patients with light-chain amyloidosis but are often attempted in
with (1) nephrotic range proteinuria; (2) fatigue, which may have a patients who have a monoclonal gammopathy and a peripheral
cardiac basis, including heart failure with preserved ejection fraction neuropathy (presumed CIDP) if amyloidosis is not considered in the
or restrictive cardiomyopathy; (3) unexplained hepatomegaly; (4) a differential diagnosis.
peripheral neuropathy resembling chronic inflammatory demyelinat- All patients with MGUS need to be monitored for life for the
ing polyneuropathy (CIDP); and (5) “atypical” multiple myeloma or development of myeloma and amyloidosis. Over 25 years, approxi-
MGUS with unexplained fatigue, weight loss, and edema. Consulting mately 25% of patients will go on to develop a more serious plasma
patients with any one of these five syndromes should lead to the cell dyscrasia. In 21%, this represents multiple myeloma, but 4% of
placement of light-chain amyloid in the differential diagnosis, and patients with MGUS will develop light-chain amyloidosis during the
screening should commence. course of observation. If screening is limited to detection of changes
Because all patients with systemic immunoglobulin light-chain consistent with multiple myeloma, such as anemia, bone pain, and
amyloidosis have a plasma cell dyscrasia, the initial step should be hypercalcemia, amyloidosis will be overlooked because all three of
screening by performing immunofixation of the serum, immunofixa- these findings are unusual in light-chain amyloidosis. Patient 2,
tion of the urine, and an immunoglobulin free light-chain assay. described above, was being followed by a hematologist for a mono-
Results of one of these three tests will be abnormal in 99% of patients clonal gammopathy, and when he developed fatigue in the absence
with light-chain amyloidosis. If results of these three tests are nega- of progressive anemia, amyloidosis was not considered.
tive, the likelihood is that one of the following conditions exists: (1) Any patient with one of the five compatible clinical syndromes
The patient does not have amyloidosis; (2) the chance of immuno- listed in Table 88.1 should be screened for monoclonal protein. If a
globulin light-chain amyloidosis is only 1%; (3) the patient has sys- monoclonal protein is not found, the likelihood of light-chain
temic amyloidosis, but it is not of immunoglobulin light-chain origin amyloidosis is very small. However, if a monoclonal gammopathy
(familial amyloid or senile systemic amyloid); or (4) the amyloidosis exists with an appropriate clinical syndrome, histologic demonstra-
is localized. tion of amyloid should be sought.
Alternatively, if a patient with nephrotic range proteinuria has a In patients who have renal, cardiac, hepatic, and peripheral nerve
monoclonal protein or an abnormal free light-chain ratio, the diag- amyloid, biopsy of the affected organs has a very high sensitivity of
nosis, which often includes minimal change glomerulopathy and demonstrating amyloid. However, these biopsies are not required if
membranoproliferative glomerulopathy, suddenly shifts to either (1) amyloid is considered in the differential diagnosis.
myeloma cast nephropathy, (2) immunoglobulin light-chain amyloi- The first diagnostic studies that should be performed in patients
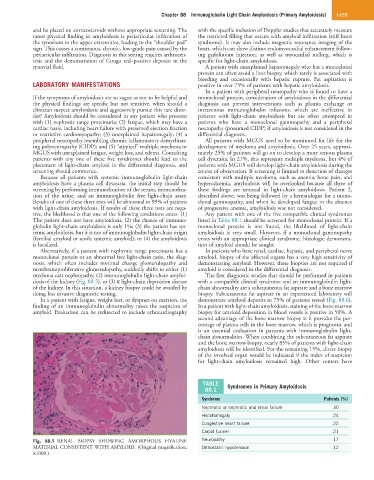
dosis of the kidney (Fig. 88.5), or (3) κ light-chain deposition disease with a compatible clinical syndrome and an immunoglobulin light-
of the kidney. In this situation, a kidney biopsy could be avoided by chain abnormality are a subcutaneous fat aspirate and a bone marrow
doing less invasive diagnostic testing. biopsy. Subcutaneous fat aspirate in an experienced laboratory will
In a patient with fatigue, weight loss, or dyspnea on exertion, the demonstrate amyloid deposits in 75% of patients tested (Fig. 88.6).
finding of an immunoglobulin abnormality raises the suspicion of In a patient with light-chain amyloidosis, staining of the bone marrow
amyloid. Evaluation can be redirected to include echocardiography biopsy for amyloid deposition in blood vessels is positive in 50%. A
second advantage of the bone marrow biopsy is it provides the per-
centage of plasma cells in the bone marrow, which is prognostic and
is an essential evaluation in patients with immunoglobulin light-
chain abnormalities. When combining the subcutaneous fat aspirate
and the bone marrow biopsy, nearly 85% of patients with light-chain
amyloidosis will be identified. For the remaining 15%, direct biopsy
of the involved organ would be indicated if the index of suspicion
for light-chain amyloidosis remained high. Other centers have
TABLE Syndromes in Primary Amyloidosis
88.1
Syndrome Patients (%)
Nephrotic or nephrotic and renal failure 30
Hepatomegaly 24
Congestive heart failure 22
Carpal tunnel 21
Fig. 88.5 RENAL BIOPSY SHOWING AMORPHOUS HYALINE Neuropathy 17
MATERIAL CONSISTENT WITH AMYLOID. (Original magnification, Orthostatic hypotension 12
×1000.)