Page 1738 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1738
Chapter 97 Graft Engineering and Cell Processing 1543
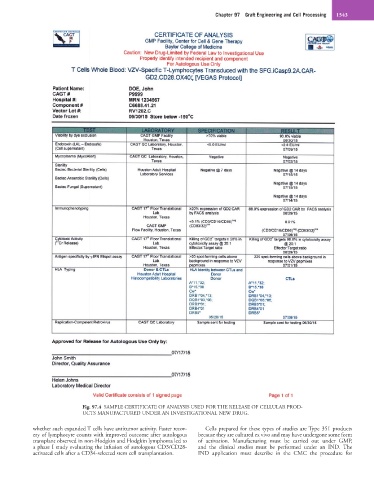
Fig. 97.4 SAMPLE CERTIFICATE OF ANALYSIS USED FOR THE RELEASE OF CELLULAR PROD-
UCTS MANUFACTURED UNDER AN INVESTIGATIONAL NEW DRUG.
whether such expanded T cells have antitumor activity. Faster recov- Cells prepared for these types of studies are Type 351 products
ery of lymphocyte counts with improved outcome after autologous because they are cultured ex vivo and may have undergone some form
transplant observed in non-Hodgkin and Hodgkin lymphoma led to of activation. Manufacturing must be carried out under GMP,
a phase I study evaluating the infusion of autologous CD3/CD28- and the clinical studies must be performed under an IND. The
activated cells after a CD34-selected stem cell transplantation. IND application must describe in the CMC the procedure for