Page 1988 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1988
1762 Part XI Transfusion Medicine
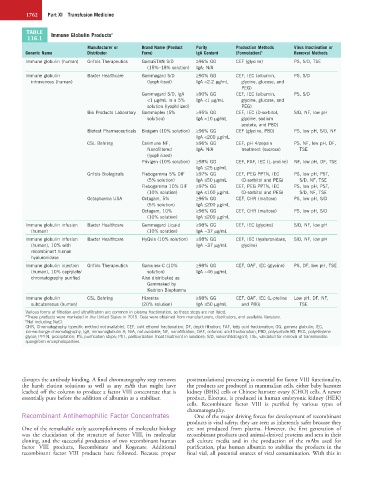
TABLE Immune Globulin Products a
116.1
Manufacturer or Brand Name (Product Purity Production Methods Virus Inactivation or
Generic Name Distributor Form) IgA Content (Formulation) b Removal Methods
Immune globulin (human) Grifols Therapeutics GamaSTAN S/D ≥96% GG CEF (glycine) PS, S/D, TSE
(15%–18% solution) IgA: N/A
Immune globulin Baxter Healthcare Gammagard S/D ≥90% GG CEF, IEC (albumin, PS, S/D
intravenous (human) (lyophilized) IgA <2.2 µg/mL glycine, glucose, and
PEG)
Gammagard S/D, IgA ≥90% GG CEF, IEC (albumin, PS, S/D
<1 µg/mL in a 5% IgA <1 µg/mL glycine, glucose, and
solution (lyophilized) PEG)
Bio Products Laboratory Gammaplex (5% >95% GG CEF, IEC (D-sorbitol, S/D, NF, low pH
solution) IgA <10 µg/mL glycine, sodium
acetate, and P80)
Biotest Pharmaceuticals Bivigam (10% solution) ≥96% GG CEF (glycine, P80) PS, low pH, S/D, NF
IgA <200 µg/mL
CSL Behring Carimune NF, ≥96% GG CEF, pH 4/pepsin PS, NF, low pH, DF,
Nanofiltered IgA: N/A treatment (sucrose) TSE
(lyophilized)
Privigen (10% solution) ≥98% GG CEF, FAF, IEC (L-proline) NF, low pH, DF, TSE
IgA ≤25 µg/mL
Grifols Biologicals Flebogamma 5% DIF ≥97% GG CEF, PEG PPTN, IEC PS, low pH, PST,
(5% solution) IgA ≤50 µg/mL (D-sorbitol and PEG) S/D, NF, TSE
Flebogamma 10% DIF ≥97% GG CEF, PEG PPTN, IEC PS, low pH, PST,
(10% solution) IgA ≤100 µg/mL (D-sorbitol and PEG) S/D, NF, TSE
Octapharma USA Octagam, 5% ≥96% GG CEF, CHR (maltose) PS, low pH, S/D
(5% solution) IgA ≤200 µg/mL
Octagam, 10% ≥96% GG CEF, CHR (maltose) PS, low pH, S/D
(10% solution) IgA ≤200 µg/mL
Immune globulin infusion Baxter Healthcare Gammagard Liquid ≥98% GG CEF, IEC (glycine) S/D, NF, low pH
(human) (10% solution) IgA ~37 µg/mL
Immune globulin infusion Baxter Healthcare HyQvia (10% solution) ≥98% GG CEF, IEC (hyaluronidase, S/D, NF, low pH
(human), 10% with IgA ~37 µg/mL glycine)
recombinant human
hyaluronidase
Immune globulin injection Grifols Therapeutics Gamunex-C (10% ≥98% GG CEF, OAF, IEC (glycine) PS, DF, low pH, TSE
(human), 10% caprylate/ solution) IgA ~46 µg/mL
chromatography purified Also distributed as
Gammaked by
Kedrion Biopharma
Immune globulin CSL Behring Hizentra ≥98% GG CEF, OAF, IEC (L-proline Low pH, DF, NF,
subcutaneous (human) (20% solution) IgA ≤50 µg/mL and P80) TSE
Various forms of filtration and ultrafiltration are common in plasma fractionation, so those steps are not listed.
a These products were marketed in the United States in 2015. Data were obtained from manufacturers, distributors, and available literature.
b Not including NaCl.
CHR, Chromatography (specific method not available); CEF, cold ethanol fractionation; DF, depth filtration; FAF, fatty acid fractionation; GG, gamma globulin; IEC,
ion-exchange chromatography; IgA, immunoglobulin A; N/A, not available; NF, nanofiltration; OAF, octanoic acid fractionation; P80, polysorbate 80; PEG, polyethylene
glycol; PPTN, precipitation; PS, purification steps; PST, pasteurization (heat treatment in solution); S/D, solvent/detergent; TSE, validated for removal of transmissible
spongiform encephalopathies.
disrupts the antibody binding. A final chromatography step removes posttranslational processing is essential for factor VIII functionality,
the harsh elution solutions as well as any mAb that might have the products are produced in mammalian cells, either baby hamster
leached off the column to produce a factor VIII concentrate that is kidney (BHK) cells or Chinese hamster ovary (CHO) cells. A newer
essentially pure before the addition of albumin as a stabilizer. product, Eloctate, is produced in human embryonic kidney (HEK)
cells. Recombinant factor VIII is purified by various types of
chromatography.
Recombinant Antihemophilic Factor Concentrates One of the major driving forces for development of recombinant
products is viral safety; they are seen as inherently safer because they
One of the remarkable early accomplishments of molecular biology are not produced from plasma. However, the first generation of
was the elucidation of the structure of factor VIII, its molecular recombinant products used animal-derived proteins and sera in their
cloning, and the successful production of two recombinant human cell culture media and in the production of the mAbs used for
factor VIII products, Recombinate and Kogenate. Additional purification, plus human albumin to stabilize the products in the
recombinant factor VIII products have followed. Because proper final vial, all potential sources of viral contamination. With this in