Page 1989 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1989
Chapter 116 Preparation of Plasma-Derived and Recombinant Human Plasma Proteins 1763
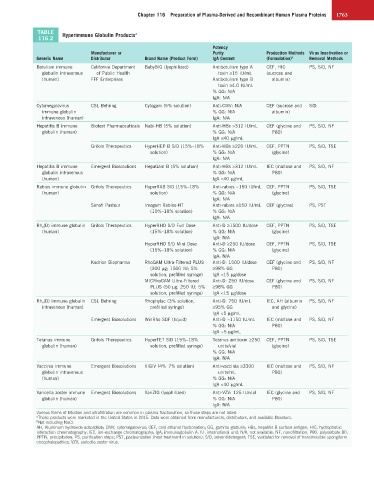
TABLE Hyperimmune Globulin Products a
116.2
Potency
Manufacturer or Purity Production Methods Virus Inactivation or
Generic Name Distributor Brand Name (Product Form) IgA Content (Formulation) b Removal Methods
Botulism immune California Department BabyBIG (lyophilized) Antibotulism type A CEF, HIC PS, S/D, NF
globulin intravenous of Public Health toxin ≥15 IU/mL (sucrose and
(human) FFF Enterprises Antibotulism type B albumin)
toxin ≥4.0 IU/mL
% GG: N/A
IgA: N/A
Cytomegalovirus CSL Behring Cytogam (5% solution) Anti-CMV: N/A CEF (sucrose and S/D
immune globulin % GG: N/A albumin)
intravenous (human) IgA: N/A
Hepatitis B immune Biotest Pharmaceuticals Nabi-HB (5% solution) Anti-HBs >312 IU/mL CEF (glycine and PS, S/D, NF
globulin (human) % GG: N/A P80)
IgA ≤40 µg/mL
Grifols Therapeutics HyperHEP B S/D (15%–18% Anti-HBs ≥220 IU/mL CEF, PPTN PS, S/D, TSE
solution) % GG: N/A (glycine)
IgA: N/A
Hepatitis B immune Emergent Biosolutions HepaGam B (5% solution) Anti-HBs >312 IU/mL IEC (maltose and PS, S/D, NF
globulin intravenous % GG: N/A P80)
(human) IgA <40 µg/mL
Rabies immune globulin Grifols Therapeutics HyperRAB S/D (15%–18% Anti-rabies ~150 IU/mL CEF, PPTN PS, S/D, TSE
(human) solution) % GG: N/A (glycine)
IgA: N/A
Sanofi Pasteur Imogam Rabies-HT Anti-rabies ≥150 IU/mL CEF (glycine) PS, PST
(10%–18% solution) % GG: N/A
IgA: N/A
Rh o (D) immune globulin Grifols Therapeutics HyperRHO S/D Full Dose Anti-D ≥1500 IU/dose CEF, PPTN PS, S/D, TSE
(human) (15%–18% solution) % GG: N/A (glycine)
IgA: N/A
HyperRHO S/D Mini Dose Anti-D ≥250 IU/dose CEF, PPTN PS, S/D, TSE
(15%–18% solution) % GG: N/A (glycine)
IgA: N/A
Kedrion Biopharma RhoGAM Ultra-Filtered PLUS Anti-D: 1500 IU/dose CEF (glycine and PS, S/D, NF
(300 µg; 1500 IU; 5% ≥98% GG P80)
solution, prefilled syringe) IgA <15 µg/dose
MICRhoGAM Ultra-Filtered Anti-D: 250 IU/dose CEF (glycine and PS, S/D, NF
PLUS (50 µg; 250 IU; 5% ≥98% GG P80)
solution, prefilled syringe) IgA <15 µg/dose
Rh o (D) immune globulin CSL Behring Rhophylac (3% solution, Anti-D: 750 IU/mL IEC, AH (albumin PS, S/D, NF
intravenous (human) prefilled syringe) ≥95% GG and glycine)
IgA <5 µg/mL
Emergent Biosolutions WinRho SDF (liquid) Anti-D ~1150 IU/mL IEC (maltose and PS, S/D, NF
% GG: N/A P80)
IgA ~5 µg/mL
Tetanus immune Grifols Therapeutics HyperTET S/D (15%–18% Tetanus antitoxin ≥250 CEF, PPTN PS, S/D, TSE
globulin (human) solution, prefilled syringe) units/vial (glycine)
% GG: N/A
IgA: N/A
Vaccinia immune Emergent Biosolutions VIGIV (4%–7% solution) Anti-vaccinia ≥3300 IEC (maltose and PS, S/D, NF
globulin intravenous units/mL P80)
(human) % GG: N/A
IgA <40 µg/mL
Varicella zoster immune Emergent Biosolutions VariZIG (lyophilized) Anti-VZV: 125 IU/vial IEC (glycine and PS, S/D, NF
globulin (human) % GG: N/A P80)
IgA: N/A
Various forms of filtration and ultrafiltration are common in plasma fractionation, so those steps are not listed.
a These products were marketed in the United States in 2015. Data were obtained from manufacturers, distributors, and available literature.
b Not including NaCl.
AH, Aluminum hydroxide adsorption; CMV, cytomegalovirus; CEF, cold ethanol fractionation; GG, gamma globulin; HBs, hepatitis B surface antigen; HIC, hydrophobic
interaction chromatography; IEC, ion-exchange chromatography; IgA, immunoglobulin A; IU, international unit; N/A, not available; NF, nanofiltration; P80, polysorbate 80;
PPTN, precipitation; PS, purification steps; PST, pasteurization (heat treatment in solution); S/D, solvent/detergent; TSE, validated for removal of transmissible spongiform
encephalopathies; VZV, varicella zoster virus.