Page 208 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 208
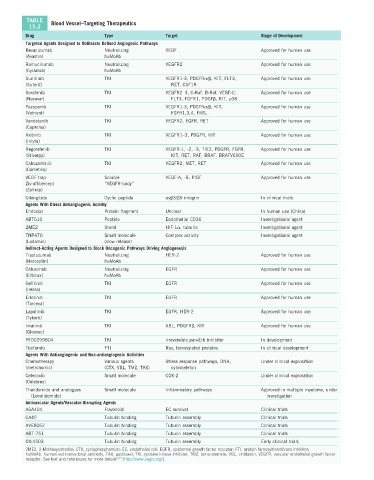
TABLE Blood Vessel–Targeting Therapeutics
15.2
Drug Type Target Stage of Development
Targeted Agents Designed to Obliterate Defined Angiogenic Pathways
Bevacizumab Neutralizing VEGF Approved for human use
(Avastin) huMoAb
Ramucirumab Neutralizing VEGFR2 Approved for human use
(Cyramza) huMoAb
Sunitinib TKI VEGFR1-3, PDGFRα/β, KIT, FLT3, Approved for human use
(Sutent) RET, CSF1R
Sorafenib TKI VEGFR2–3, C-Raf, B-Raf, VEGF-C, Approved for human use
(Nexavar) FLT3, FGFR1, PDGFβ, KIT, p38
Pazopanib TKI VEGFR1-3, PDGFRα/β, KIT, Approved for human use
(Votrient) FGFR1,3,4, FMS,
Vandetanib TKI VEGFR2, EGFR, RET Approved for human use
(Caprelsa)
Axitinib TKI VEGFR1–3, PDGFR, KIT Approved for human use
(Inlyta)
Regorafenib TKI VEGFR-1, -2, -3, TIE2, PDGFR, FGFR, Approved for human use
(Stivarga) KIT, RET, RAF, BRAF, BRAFV600E
Cabozantinib TKI VEGFR2, MET, RET Approved for human use
(Cometriq)
VEGF-trap Soluble VEGF-A, -B, PlGF Approved for human use
Ziv-aflibercept “VEGFR-body”
(Zaltrap)
Cilengitide Cyclic peptide αvβ3/β5 integrin In clinical trials
Agents With Direct Antiangiogenic Activity
Endostar Protein fragment Unclear In human use (China)
ABT510 Peptide Endothelial CD36 Investigational agent
2ME2 Sterol HIF-1α, tubulin Investigational agent
TNP470 Small molecule Complex activity Investigational agent
(Lodamin) (slow release)
Indirect-Acting Agents Designed to Block Oncogenic Pathways Driving Angiogenesis
Trastuzumab Neutralizing HER-2 Approved for human use
(Herceptin) huMoAb
Cetuximab Neutralizing EGFR Approved for human use
(Erbitux) huMoAb
Gefitinib TKI EGFR Approved for human use
(Iressa)
Erlotinib TKI EGFR Approved for human use
(Tarceva)
Lapatinib TKI EGFR, HER-2 Approved for human use
(Tykerb)
Imatinib TKI ABL, PDGFRβ, KIT Approved for human use
(Gleevec)
PF00299804 TKI Irreversible pan-Erb inhibitor In development
Tipifarnib FTI Ras, farnesylated proteins In clinical development
Agents With Antiangiogenic and Non-antiangiogenic Activities
Chemotherapy Various agents Stress response pathways, DNA, Under clinical exploration
(metronomic) (CTX, VBL, TMZ, TAX) cytoskeleton
Celecoxib Small molecule COX-2 Under clinical exploration
(Celebrex)
Thalidomide and analogues Small molecule Inflammatory pathways Approved in multiple myeloma, under
(Lenalidomide) investigation
Antivascular Agents/Vascular-Disrupting Agents
ASA404 Flavonoid EC survival Clinical trials
CA4P Tubulin binding Tubulin assembly Clinical trials
AVE8062 Tubulin binding Tubulin assembly Clinical trials
ABT-751 Tubulin binding Tubulin assembly Clinical trials
OXi4503 Tubulin binding Tubulin assembly Early clinical trials
2ME2, 2-Methoxyestradiol; CTX, cyclophosphamide; EC, endothelial cell; EGFR, epidermal growth factor receptor; FTI, protein farnesyltransferase inhibitor;
huMoAb, humanized monoclonal antibody; TAX, paclitaxel; TKI, tyrosine kinase inhibitor; TMZ, temozolomide; VBL, vinblastin; VEGFR, vascular endothelial growth factor
receptor. See text and references for more details 2,34 (http://www.angio.org/).