Page 2096 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2096
Chapter 124 Megakaryocyte and Platelet Structure 1859
A B C D E
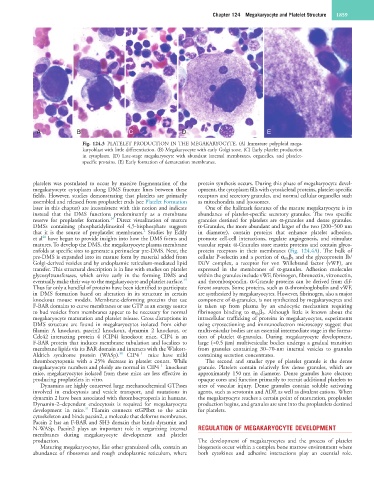
Fig. 124.3 PLATELET PRODUCTION IN THE MEGAKARYOCYTE. (A) Immature polyploid mega-
karyoblast with little differentiation. (B) Megakaryocyte with early Golgi zone. (C) Early platelet production
in cytoplasm. (D) Late-stage megakaryocyte with abundant internal membranes, organelles, and platelet-
specific proteins. (E) Early formation of demarcation membranes.
platelets was postulated to occur by massive fragmentation of the protein synthesis occurs. During this phase of megakaryocyte devel-
megakaryocyte cytoplasm along DMS fracture lines between these opment, the cytoplasm fills with cytoskeletal proteins, platelet-specific
fields. However, studies demonstrating that platelets are primarily receptors and secretory granules, and normal cellular organelles such
assembled and released from proplatelet ends (see Platelet Formation as mitochondria and lysosomes.
later in this chapter) are inconsistent with this notion and indicate One of the hallmark features of the mature megakaryocyte is its
instead that the DMS functions predominantly as a membrane abundance of platelet-specific secretory granules. The two specific
24
reserve for proplatelet formation. Direct visualization of mature granules destined for platelets are α-granules and dense granules.
DMSs containing phosphatidylinositol 4,5-bisphosphate suggests α-Granules, the more abundant and larger of the two (200–500 nm
2
that it is the source of proplatelet membranes. Studies by Eckly in diameter), contain proteins that enhance platelet adhesion,
28
et al have begun to provide insights into how the DMS forms and promote cell-cell interactions, regulate angiogenesis, and stimulate
matures. To develop the DMS, the megakaryocyte plasma membrane vascular repair. α-Granules store matrix proteins and contain glyco-
enfolds at specific sites to generate a perinuclear pre-DMS. Next, the protein receptors in their membranes (Fig. 124.4A). The bulk of
pre-DMS is expanded into its mature form by material added from cellular P-selectin and a portion of α IIb β 3 and the glycoprotein Ib/
Golgi-derived vesicles and by endoplasmic reticulum-mediated lipid IX/V complex, a receptor for von Willebrand factor (vWF), are
transfer. This structural description is in line with studies on platelet expressed in the membranes of α-granules. Adhesion molecules
glycosyltransferases, which arrive early in the forming DMS and within the granules include vWF, fibrinogen, fibronectin, vitronectin,
29
eventually make their way to the megakaryocyte and platelet surface. and thrombospondin. α-Granule proteins can be derived from dif-
Thus far only a handful of proteins have been identified to participate ferent sources. Some proteins, such as α-thromboglobulin and vWF,
in DMS formation based on alteration in its structure in certain are synthesized by megakaryocytes. However, fibrinogen, also a major
knockout mouse models. Membrane-deforming proteins that use component of α-granules, is not synthesized by megakaryocytes and
F-BAR domains to curve membranes or use GTP as an energy source is taken up from plasma by an endocytic mechanism requiring
to bud vesicles from membranes appear to be necessary for normal fibrinogen binding to α IIb β 3 . Although little is known about the
megakaryocyte maturation and platelet release. Gross disruptions in intracellular trafficking of proteins in megakaryocytes, experiments
DMS structure are found in megakaryocytes isolated from either using cryosectioning and immunoelectron microscopy suggest that
filamin A knockout, pascin2 knockout, dynamin 2 knockout, or multivesicular bodies are an essential intermediate stage in the forma-
Cdc42 interacting protein 4 (CIP4) knockout mice. CIP4 is an tion of platelet α-granules. During megakaryocyte development,
F-BAR protein that induces membrane tubulation and localizes to large (≈0.5 µm) multivesicular bodies undergo a gradual transition
membrane lipids via its BAR domain and interacts with the Wiskott- from granules containing 30–70-nm internal vesicles to granules
−/−
30
Aldrich syndrome protein (WASp). CIP4 mice have mild containing secretion concentrates.
thrombocytopenia with a 25% decrease in platelet counts. While The second and smaller type of platelet granule is the dense
−/−
megakaryocyte numbers and ploidy are normal in CIP4 knockout granule. Platelets contain relatively few dense granules, which are
mice, megakaryocytes isolated from these mice are less effective in approximately 150 nm in diameter. Dense granules have electron
producing proplatelets in vitro. opaque cores and function primarily to recruit additional platelets to
Dynamins are highly conserved large mechanochemical GTPases sites of vascular injury. Dense granules contain soluble activating
involved in endocytosis and vesicle transport, and mutations in agents, such as serotonin and ADP, as well as divalent cations. When
dynamin 2 have been associated with thrombocytopenia in humans. the megakaryocyte reaches a certain point of maturation, proplatelet
Dynamin-2–dependent endocytosis is required for megakaryocyte production begins, and granules are sent into the proplatelets destined
31
development in mice. Filamin connects αGPIbα to the actin for platelets.
cytoskeleton and binds pacsin2, a molecule that deforms membranes.
Pacsin 2 has an F-BAR and SH3 domain that binds dynamin and
N-WASp. Pacsin2 plays an important role in organizing internal REGULATION OF MEGAKARYOCYTE DEVELOPMENT
membranes during megakaryocyte development and platelet
production. The development of megakaryocytes and the process of platelet
Maturing megakaryocytes, like other granulated cells, contain an biogenesis occur within a complex bone marrow environment where
abundance of ribosomes and rough endoplasmic reticulum, where both cytokines and adhesive interactions play an essential role.