Page 2099 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2099
1862 Part XII Hemostasis and Thrombosis
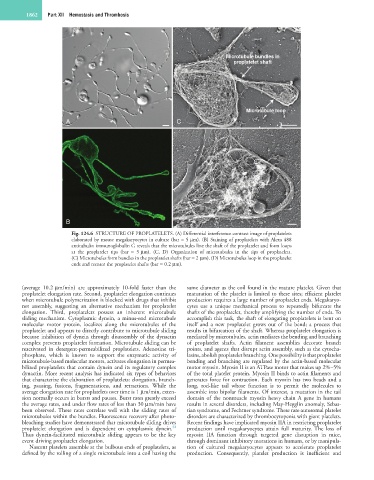
Microtubule bundles in
proplatelet shaft
Microtubule loop
A C
B D
Fig. 124.6 STRUCTURE OF PROPLATELETS. (A) Differential interference contrast image of proplatelets
elaborated by mouse megakaryocytes in culture (bar = 5 µm). (B) Staining of proplatelets with Alexa 488
antitubulin immunoglobulin G reveals that the microtubules line the shaft of the proplatelet and form loops
at the proplatelet tips (bar = 5 µm). (C, D) Organization of microtubules in the tips of proplatelets.
(C) Microtubules form bundles in the proplatelet shafts (bar = 2 µm). (D) Microtubules loop in the proplatelet
ends and reenter the proplatelet shafts (bar = 0.2 µm).
(average 10.2 µm/min) are approximately 10-fold faster than the same diameter as the coil found in the mature platelet. Given that
proplatelet elongation rate. Second, proplatelet elongation continues maturation of the platelet is limited to these sites; efficient platelet
when microtubule polymerization is blocked with drugs that inhibit production requires a large number of proplatelet ends. Megakaryo-
net assembly, suggesting an alternative mechanism for proplatelet cytes use a unique mechanical process to repeatedly bifurcate the
elongation. Third, proplatelets possess an inherent microtubule shafts of the proplatelet, thereby amplifying the number of ends. To
sliding mechanism. Cytoplasmic dynein, a minus-end microtubule accomplish this task, the shaft of elongating proplatelets is bent on
molecular motor protein, localizes along the microtubules of the itself and a new proplatelet grows out of the bend; a process that
proplatelet and appears to directly contribute to microtubule sliding results in bifurcation of the shaft. Whereas proplatelet elongation is
because inhibition of dynein through disassembly of the dynactin mediated by microtubules, actin mediates the bending and branching
complex prevents proplatelet formation. Microtubule sliding can be of proplatelet shafts. Actin filament assemblies decorate branch
reactivated in detergent-permeabilized proplatelets. Adenosine tri- points, and agents that disrupt actin assembly, such as the cytocha-
phosphate, which is known to support the enzymatic activity of lasins, abolish proplatelet branching. One possibility is that proplatelet
microtubule-based molecular motors, activates elongation in permea- bending and branching are regulated by the actin-based molecular
bilized proplatelets that contain dynein and its regulatory complex motor myosin. Myosin II is an ATPase motor that makes up 2%–5%
dynactin. More recent analysis has indicated six types of behaviors of the total platelet protein. Myosin II binds to actin filaments and
that characterize the elaboration of proplatelets: elongation, branch- generates force for contraction. Each myosin has two heads and a
ing, pausing, fusions, fragmentations, and retractions. While the long, rod-like tail whose function is to permit the molecules to
average elongation rate for proplatelets over time is 1 µm/min, exten- assemble into bipolar filaments. Of interest, a mutation in the tail
sion normally occurs in bursts and pauses. Burst rates greatly exceed domain of the nonmuscle myosin heavy chain A gene in humans
the average rates, and under flow rates of less than 30 µm/min have results in several disorders, including May-Hegglin anomaly, Sebas-
been observed. These rates correlate well with the sliding rates of tian syndrome, and Fechtner syndrome. These rare autosomal platelet
microtubules within the bundles. Fluorescence recovery after photo- disorders are characterized by thrombocytopenia with giant platelets.
bleaching studies have demonstrated that microtubule sliding drives Recent findings have implicated myosin IIA in restricting proplatelet
32
proplatelet elongation and is dependent on cytoplasmic dynein. production until megakaryocytes attain full maturity. The loss of
Thus dynein-facilitated microtubule sliding appears to be the key myosin IIA function through targeted gene disruption in mice,
event driving proplatelet elongation. through dominant inhibitory mutations in humans, or by manipula-
Nascent platelets assemble at the bulbous ends of proplatelets, as tion of cultured megakaryocytes appears to accelerate proplatelet
defined by the rolling of a single microtubule into a coil having the production. Consequently, platelet production is inefficient and