Page 2110 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2110
Chapter 125 Molecular Basis of Platelet Function 1873
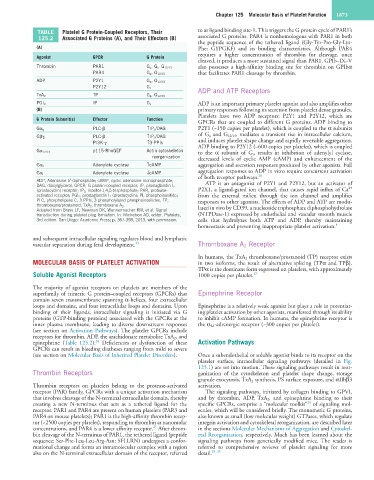
TABLE Platelet G Protein-Coupled Receptors, Their to as ligand binding site-1. This triggers the G protein cycle of PAR1’s
125.2 Associated G Proteins (A), and Their Effectors (B) associated G proteins. PAR4 is nonhomologous with PAR1 in both
the peptide sequence of the tethered ligand (Gly-Tyr-Pro-Gly-Lys-
(A) Phe; GYPGKF) and its binding characteristics. Although PAR4
requires a higher concentration of thrombin for cleavage, once
Agonist GPCR G Protein
cleaved, it produces a more sustained signal than PAR1. GPIb–IX–V
Thrombin PAR1 G q , G i , G 12/13 also possesses a high-affinity binding site for thrombin on GPIbα
PAR4 G q , G 12/13 that facilitates PAR1 cleavage by thrombin.
ADP P2Y1 G q , G 12/13
P2Y12 G i ADP and ATP Receptors
TP
TxA 2 G q , G 12/13
IP
PGI 2 G s ADP is an important primary platelet agonist and also amplifies other
(B) primary responses following its secretion from platelet dense granules.
Platelets have two ADP receptors: P2Y1 and P2Y12, which are
G Protein Subunit(s) Effector Function
GPCRs that are coupled to different G proteins. ADP binding to
PLC-β ↑IP 3 /DAG P2Y1 (~150 copies per platelet), which is coupled to the α subunits
Gα q
PLC-β ↑IP 3 /DAG of G q and G 12/13 , mediates a transient rise in intracellular calcium,
Gβγ i and induces platelet shape change and rapidly reversible aggregation.
PI3K-γ ↑3-PPIs
ADP binding to P2Y12 (~600 copies per platelet), which is coupled
p115-RhoGEF Actin cytoskeleton
Gα 12/13 to the α subunit of G i , results in inhibition of adenylyl cyclase,
reorganization decreased levels of cyclic AMP (cAMP) and enhancement of the
Adenylate cyclase ↑cAMP aggregation and secretion responses produced by other agonists. Full
Gα s
Adenylate cyclase ↓cAMP aggregation responses to ADP in vitro require concurrent activation
Gα i 26
of both receptor pathways.
ADP, Adenosine 5′-diphosphate; cAMP, cyclic adenosine monophosphate; ATP is an antagonist of P2Y1 and P2Y12, but an activator of
DAG, diacylglycerol; GPCR; G protein-coupled receptor; IP, prostaglandin I 2 2+
(prostacyclin) receptor; IP 3 , inositol-1,4,5-trisphosphate; PAR, protease- P2X1, a ligand-gated ion channel, that causes rapid influx of Ca
activated receptor; PGI 2 , prostaglandin I 2 (prostacyclin); PI, phosphoinositide; from the external milieu through the ion channel and amplifies
PLC, phospholipase C; 3-PPIs, 3-phosphorylated phosphoinositides; TP, responses to other agonists. The effects of ADP and ATP are modu-
thromboxane/prostanoid; TxA 2 , thromboxane A 2 . lated in vivo by CD39, a nucleoside triphosphate diphosphohydrolase
Adapted from Brass LF, Newman DK, Wannermacher KM, et al: Signal
transduction during platelet plug formation. In: Michelson AD, editor. Platelets, (NTPDase-1) expressed by endothelial and vascular smooth muscle
3rd edition. San Diego: Academic Press; p. 367-398, 2013, with permission. cells that hydrolyzes both ATP and ADP, thereby maintaining
homeostasis and preventing inappropriate platelet activation. 4
and subsequent intracellular signaling regulates blood and lymphatic
vascular separation during fetal development. 23 Thromboxane A 2 Receptor
In humans, the TxA 2 thromboxane/prostanoid (TP) receptor exists
MOLECULAR BASIS OF PLATELET ACTIVATION in two isoforms, the result of alternative splicing (TPα and TPβ).
TPα is the dominant form expressed on platelets, with approximately
Soluble Agonist Receptors 1000 copies per platelet. 27
The majority of agonist receptors on platelets are members of the
superfamily of trimeric G protein–coupled receptors (GPCRs) that Epinephrine Receptor
contain seven transmembrane spanning α-helices, four extracellular
loops and domains, and four intracellular loops and domains. Upon Epinephrine is a relatively weak agonist but plays a role in potentiat-
binding of their ligands, intracellular signaling is initiated via G ing platelet activation by other agonists, manifested through its ability
proteins (GTP-binding proteins) associated with the GPCRs at the to inhibit cAMP formation. In humans, the epinephrine receptor is
inner plasma membrane, leading to diverse downstream responses the α 2A -adrenergic receptor (~300 copies per platelet).
(see section on Activation Pathways). The platelet GPCRs include
receptors for thrombin, ADP, the arachidonate metabolite TxA 2 , and
24
epinephrine (Table 125.2). Deficiencies or dysfunction of these Activation Pathways
GPCRs can result in bleeding diatheses ranging from mild to severe
(see section on Molecular Basis of Inherited Platelet Disorders). Once a subendothelial or soluble agonist binds to its receptor on the
platelet surface, intracellular signaling pathways (detailed in Fig.
125.1) are set into motion. These signaling pathways result in reor-
Thrombin Receptors ganization of the cytoskeleton and platelet shape change, storage
granule exocytosis, TxA 2 synthesis, PS surface exposure, and αIIbβ3
Thrombin receptors on platelets belong to the protease-activated activation.
receptor (PAR) family, GPCRs with a unique activation mechanism The signaling pathways, initiated by collagen binding to GPVI,
that involves cleavage of the N-terminal extracellular domain, thereby and by thrombin, ADP, TxA 2 , and epinephrine binding to their
28
creating a new N-terminus that acts as a tethered ligand for the specific GPCRs, comprise a “molecular toolkit” of signaling mol-
receptor. PAR1 and PAR4 are present on human platelets (PAR3 and ecules, which will be considered briefly. The monomeric G proteins,
PAR4 on mouse platelets); PAR1 is the high-affinity thrombin recep- also known as small (low molecular weight) GTPases, which regulate
tor (~2500 copies per platelet), responding to thrombin at nanomolar integrin activation and cytoskeletal reorganization, are described later
25
concentrations, and PAR4 is a lower affinity receptor. After throm- in the sections Molecular Mechanisms of Aggregation and Cytoskel-
bin cleavage of the N-terminus of PAR1, the tethered ligand (peptide etal Reorganization, respectively. Much has been learned about the
sequence: Ser-Phe-Leu-Leu-Arg-Asn; SFLLRN) undergoes a confor- signaling pathways from genetically modified mice. The reader is
mational change and forms an intramolecular complex with a region referred to comprehensive reviews of platelet signaling for more
also on the N-terminal extracellular domain of the receptor, referred detail. 28–35