Page 2230 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2230
Chapter 133 Heparin-Induced Thrombocytopenia 1977
stimulation, as well as the inclusion of negative and positive HIT sera Three enzyme(-linked) immuno(sorbent) assays (EIAs or ELISAs)
of variable reactivity to ensure that the test platelets identify weaker are commercially available for detecting antibodies that recognize PF4
HIT sera. The characteristic activation profile produced by HIT bound to heparin, to the polyanion, polyvinyl sulfonate, or to PF4
serum includes increased platelet activation at low heparin concentra- (from platelet lysate) bound to protamine. These assays are very sensi-
tions (0.05–0.3 units/mL), but background platelet activation at high tive for detecting HIT antibodies but are much more likely than
9
heparin concentrations (10–100 units/mL). Strong platelet activa- washed platelet activation assays to detect clinically insignificant
tion induced by HIT sera even in the absence of pharmacologic anti-PF4/heparin antibodies. 2,3,5 Thus results of laboratory tests must
heparin is a feature of delayed-onset HIT and may generally indicate be interpreted in the clinical context—that is, HIT is a “clinicopatho-
4
more severe HIT. 18 logic” syndrome. Activation and antigen assays are not 100% con-
cordant, and there are advantages if reference laboratories are able to
perform both types of assay. In general, serum or plasma from patients
with clinical HIT have strongly positive assay results, whereas clini-
2,5
cally insignificant antibodies give weaker results. Indeed, if the EIA
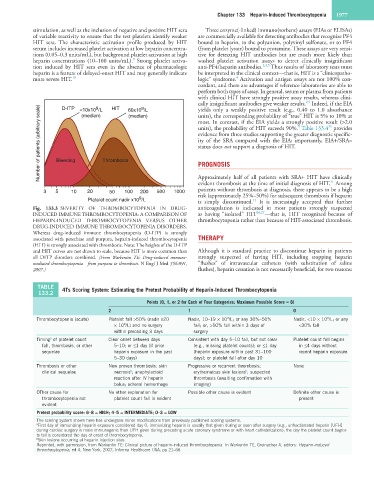
Number of patients (arbitrary scale) Bleeding Thrombosis units), the probability of HIT exceeds 90%. Table 133.4 provides
D-ITP
HIT
9
9
yields only a weakly positive result (e.g., 0.40 to 1.0 absorbance
<10x10 /L
60x10 /L
(median)
(median)
units), the corresponding probability of “true” HIT is 5% to 10% at
most. In contrast, if the EIA yields a strongly positive result (>2.0
5
25
evidence from three studies supporting the greater diagnostic specific-
ity of the SRA compared with the EIA: importantly, EIA+/SRA–
status does not support a diagnosis of HIT.
PROGNOSIS
Approximately half of all patients with SRA+ HIT have clinically
11
evident thrombosis at the time of initial diagnosis of HIT. Among
3 5 10 20 50 100 200 500 1000 patients without thrombosis at diagnosis, there appears to be a high
risk (approximately 25%–50%) for subsequent thrombosis if heparin
9
Platelet count nadir x10 /L is simply discontinued. It is increasingly accepted that further
11
Fig. 133.3 SEVERITY OF THROMBOCYTOPENIA IN DRUG- anticoagulation is indicated in most patients strongly suspected
INDUCED IMMUNE THROMBOCYTOPENIA: A COMPARISON OF as having “isolated” HIT 26,27 —that is, HIT recognized because of
HEPARIN-INDUCED THROMBOCYTOPENIA VERSUS OTHER thrombocytopenia rather than because of HIT-associated thrombosis.
DRUG-INDUCED IMMUNE THROMBOCYTOPENIA DISORDERS.
Whereas drug-induced immune thrombocytopenia (D-ITP) is strongly
associated with petechiae and purpura, heparin-induced thrombocytopenia THERAPY
(HIT) is strongly associated with thrombosis. Note: The heights of the D-ITP
and HIT curves are not drawn to scale, because HIT is more common than Although it is standard practice to discontinue heparin in patients
all DITP disorders combined. (From Warkentin TE: Drug-induced immune- strongly suspected of having HIT, including stopping heparin
mediated thrombocytopenia—from purpura to thrombosis. N Engl J Med 356:891, “flushes” of intravascular catheters (with substitution of saline
2007.) flushes), heparin cessation is not necessarily beneficial, for two reasons:
TABLE 4Ts Scoring System: Estimating the Pretest Probability of Heparin-Induced Thrombocytopenia
133.2
Points (0, 1, or 2 for Each of Four Categories: Maximum Possible Score = 8)
2 1 0
Thrombocytopenia (acute) Platelet fall >50% (nadir ≥20 Nadir, 10–19 × 10 /L; or any 30%–50% Nadir, <10 × 10 /L; or any
9
9
× 10 /L) and no surgery fall; or, >50% fall within 3 days of <30% fall
9
within preceding 3 days surgery
a
Timing of platelet count Clear onset between days Consistent with day 5–10 fall, but not clear Platelet count fall begins
fall, thrombosis, or other 5–10; or ≤1 day (if prior (e.g., missing platelet counts); or ≤1 day in ≤4 days without
sequelae heparin exposure in the past (heparin exposure within past 31–100 recent heparin exposure
5–30 days) days); or platelet fall after day 10
Thrombosis or other New proven thrombosis; skin Progressive or recurrent thrombosis; None
b
b
clinical sequelae necrosis ; anaphylactoid erythematous skin lesions ; suspected
reaction after IV heparin thrombosis (awaiting confirmation with
bolus; adrenal hemorrhage imaging)
OTher cause for No other explanation for Possible other cause is evident Definite other cause is
thrombocytopenia not platelet count fall is evident present
evident
Pretest probability score: 6–8 = HIGH; 4–5 = INTERMEDIATE; 0–3 = LOW
The scoring system shown here has undergone minor modifications from previously published scoring systems.
a First day of immunizing heparin exposure considered day 0; immunizing heparin is usually that given during or soon after surgery (e.g., unfractionated heparin [UFH]
during cardiac surgery is more immunogenic than UFH given during preceding acute coronary syndrome or with heart catheterization); the day the platelet count begins
to fall is considered the day of onset of thrombocytopenia.
b Skin lesions occurring at heparin injection sites.
Reprinted, with permission, from Warkentin TE: Clinical picture of heparin-induced thrombocytopenia. In Warkentin TE, Greinacher A, editors: Heparin-induced
thrombocytopenia, ed 4, New York, 2007, Informa Healthcare USA, pp 21–66.