Page 2231 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2231
1978 Part XII Hemostasis and Thrombosis
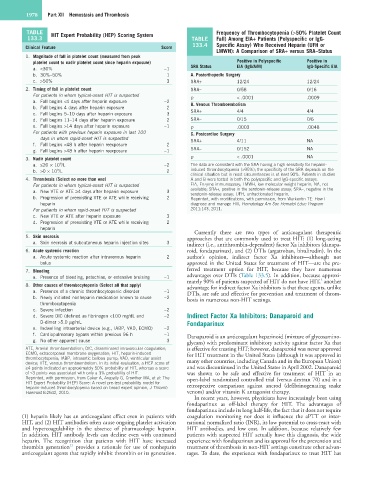
TABLE HIT Expert Probability (HEP) Scoring System Frequency of Thrombocytopenia (>50% Platelet Count
133.3 TABLE Fall) Among EIA+ Patients (Polyspecific or IgG-
133.4 Specific Assay) Who Received Heparin (UFH or
Clinical Feature Score
LMWH): A Comparison of SRA+ versus SRA–Status
1. Magnitude of fall in platelet count (measured from peak
platelet count to nadir platelet count since heparin exposure) Positive in Polyspecific Positive in
a. <30% −1 SRA Status EIA (IgG/A/M) IgG-Specific EIA
b. 30%–50% 1 A. Postorthopedic Surgery
c. >50% 3 SRA+ 12/24 12/24
2. Timing of fall in platelet count SRA– 0/58 0/16
For patients in whom typical-onset HIT is suspected p < .0001 .0009
a. Fall begins <4 days after heparin exposure −2 B. Venous Thromboembolism
b. Fall begins 4 days after heparin exposure 2 SRA+
c. Fall begins 5–10 days after heparin exposure 3 4/4 4/4
d. Fall begins 11–14 days after heparin exposure 2 SRA– 0/15 0/6
e. Fall begins >14 days after heparin exposure −1 p .0003 .0048
For patients with previous heparin exposure in last 100 C. Postcardiac Surgery
days in whom rapid-onset HIT is suspected SRA+ 4/11 NA
f. Fall begins <48 h after heparin reexposure 2
g. Fall begins >48 h after heparin reexposure −1 SRA– 0/152 NA
3. Nadir platelet count p < .0001 NA
a. ≤20 × 10 /L −2 The data are consistent with the SRA having a high sensitivity for heparin-
9
9
b. >0 × 10 /L 2 induced thrombocytopenia (>95%); the specificity of the SRA depends on the
clinical situation but in most circumstances is at least 90%. Patients in studies
4. Thrombosis (Select no more than one) A and B were tested in both the polyspecific and IgG-specific assays.
For patients in whom typical-onset HIT is suspected EIA, Enzyme immunoassay; LMWH, low-molecular-weight heparin; NA, not
a. New VTE or ATE ≥4 days after heparin exposure 3 available; SRA+, positive in the serotonin-release assay; SRA–, negative in the
serotonin-release assay; UFH, unfractionated heparin.
b. Progression of preexisting VTE or ATE while receiving 2 Reprinted, with modifications, with permission, from Warkentin TE: How I
heparin diagnose and manage HIT, Hematology Am Soc Hematol Educ Program
For patients in whom rapid-onset HIT is suspected 2011:143, 2011.
c. New VTE or ATE after heparin exposure 3
d. Progression of preexisting VTE or ATE while receiving 2
heparin
Currently there are two types of anticoagulant therapeutic
5. Skin necrosis approaches that are commonly used to treat HIT: (1) long-acting
a. Skin necrosis at subcutaneous heparin injection sites 3 indirect (i.e., antithrombin-dependent) factor Xa inhibitors (danapa-
6. Acute systemic reaction roid, fondaparinux), and (2) DTIs (argatroban, bivalirudin). In the
a. Acute systemic reaction after intravenous heparin 2 author’s opinion, indirect factor Xa inhibitors—although not
bolus approved in the United States for treatment of HIT—are the pre-
7. Bleeding ferred treatment option for HIT, because they have numerous
a. Presence of bleeding, petechiae, or extensive bruising −1 advantages over DTIs (Table 133.5). In addition, because approxi-
5
8. Other causes of thrombocytopenia (Select all that apply) mately 90% of patients suspected of HIT do not have HIT, another
advantage for indirect factor Xa inhibitors is that these agents, unlike
a. Presence of a chronic thrombocytopenic disorder −1 DTIs, are safe and effective for prevention and treatment of throm-
b. Newly initiated nonheparin medication known to cause −2 bosis in numerous non-HIT settings.
thrombocytopenia
c. Severe infection −2
d. Severe DIC (defined as fibrinogen <100 mg/dL and −2 Indirect Factor Xa Inhibitors: Danaparoid and
D-dimer >5.0 µg/mL Fondaparinux
e. Indwelling intraarterial device (e.g., IABP, VAD, ECMO) −2
f. Cardiopulmonary bypass within previous 96 h −1 Danaparoid is an anticoagulant heparinoid (mixture of glycosamino-
g. No other apparent cause 3 glycans) with predominant inhibitory activity against factor Xa that
ATE, Arterial thromboembolism; DIC, disseminated intravascular coagulation; is effective for treating HIT; however, danaparoid was never approved
ECMO, extracorporeal membrane oxygenation; HIT, heparin-induced for HIT treatment in the United States (although it was approved in
thrombocytopenia; IABP, intraaortic balloon pump; VAD, ventricular assist many other countries, including Canada and in the European Union)
device; VTE, venous thromboembolism. In its initial evaluation, a HEP score of
≥4 points indicated an approximately 50% probability of HIT, whereas a score and was discontinued in the United States in April 2002. Danaparoid
of ≤3 points was associated with only a 3% probability of HIT. was shown to be safe and effective for treatment of HIT in an
Reprinted, with permission, from Cuker A, Arepally G, Crowther MA, et al: The open-label randomized controlled trial (versus dextran 70) and in a
HIT Expert Probability (HEP) Score: A novel pre-test probability model for retrospective comparison against ancrod (defibrinogenating snake
heparin-induced thrombocytopenia based on broad expert opinion, J Thromb
Haemost 8:2642, 2010. venom) and/or vitamin K antagonist therapy.
In recent years, however, physicians have increasingly been using
fondaparinux as off-label therapy for HIT. The advantages of
fondaparinux include its long half-life, the fact that it does not require
(1) heparin likely has an anticoagulant effect even in patients with coagulation monitoring nor does it influence the aPTT or inter-
HIT, and (2) HIT antibodies often cause ongoing platelet activation national normalized ratio (INR), its low potential to cross-react with
and hypercoagulability in the absence of pharmacologic heparin. HIT antibodies, and low cost. In addition, because relatively few
In addition, HIT antibody levels can decline even with continued patients with suspected HIT actually have this diagnosis, the wide
heparin. The recognition that patients with HIT have increased experience with fondaparinux and its approval for the prevention and
12
thrombin generation provides a rationale for use of nonheparin treatment of thrombosis in non-HIT settings constitute other advan-
anticoagulant agents that rapidly inhibit thrombin or its generation. tages. To date, the experience with fondaparinux to treat HIT has