Page 2436 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2436
2174 Part XII Hemostasis and Thrombosis
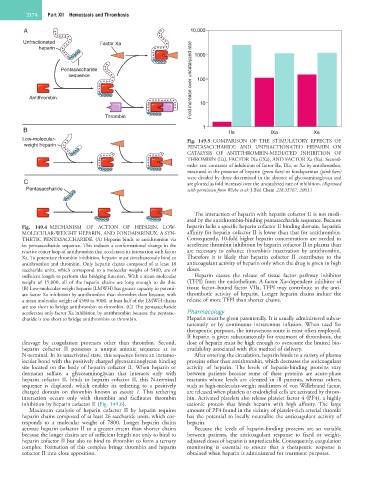
A 10,000
Unfractionated Factor Xa
Fold increase over uncatalyzed rate 100
heparin 1000
Pentasaccharide
sequence
Antithrombin 10
Thrombin
B 1 IIa IXa Xa
Low-molecular- Fig. 149.5 COMPARISON OF THE STIMULATORY EFFECTS OF
weight heparin PENTASACCHARIDE AND UNFRACTIONATED HEPARIN ON
CATALYSIS OF ANTITHROMBIN-MEDIATED INHIBITION OF
THROMBIN (IIa), FACTOR IXa (IXa), AND FACTOR Xa (Xa). Second-
order rate constants of inhibition of factor IIa, IXa, or Xa by antithrombin,
measured in the presence of heparin (green bars) or fondaparinux (pink bars)
were divided by those determined in the absence of glycosaminoglycan and
C are plotted as fold increases over the uncatalyzed rate of inhibition. (Reprinted
Pentasaccharide with permission from Wiebe et al: J Biol Chem 228:35767, 2003.)
The interaction of heparin with heparin cofactor II is not medi-
ated by the antithrombin-binding pentasaccharide sequence. Because
Fig. 149.4 MECHANISM OF ACTION OF HEPARIN, LOW- heparin lacks a specific heparin cofactor II binding domain, heparin’s
MOLECULAR-WEIGHT HEPARIN, AND FONDAPARINUX, A SYN- affinity for heparin cofactor II is lower than that for antithrombin.
THETIC PENTASACCHARIDE. (A) Heparin binds to antithrombin via Consequently, 10-fold higher heparin concentrations are needed to
its pentasaccharide sequence. This induces a conformational change in the accelerate thrombin inhibition by heparin cofactor II in plasma than
reactive center loop of antithrombin that accelerates its interaction with factor are necessary to enhance thrombin’s inactivation by antithrombin.
Xa. To potentiate thrombin inhibition, heparin must simultaneously bind to Therefore it is likely that heparin cofactor II contributes to the
antithrombin and thrombin. Only heparin chains composed of at least 18 anticoagulant activity of heparin only when the drug is given in high
saccharide units, which correspond to a molecular weight of 5400, are of doses.
sufficient length to perform this bridging function. With a mean molecular Heparin causes the release of tissue factor pathway inhibitor
weight of 15,000, all of the heparin chains are long enough to do this. (TFPI) from the endothelium. A factor Xa–dependent inhibitor of
(B) Low-molecular-weight heparin (LMWH) has greater capacity to potenti- tissue factor–bound factor VIIa, TFPI may contribute to the anti-
ate factor Xa inhibition by antithrombin than thrombin does because, with thrombotic activity of heparin. Longer heparin chains induce the
a mean molecular weight of 4500 to 5000, at least half of the LMWH chains release of more TFPI than shorter chains.
are too short to bridge antithrombin to thrombin. (C) The pentasaccharide
accelerates only factor Xa inhibition by antithrombin because the pentasac- Pharmacology
charide is too short to bridge antithrombin to thrombin. Heparin must be given parenterally. It is usually administered subcu-
taneously or by continuous intravenous infusion. When used for
therapeutic purposes, the intravenous route is most often employed.
If heparin is given subcutaneously for treatment of thrombosis, the
cleavage by coagulation proteases other than thrombin. Second, dose of heparin must be high enough to overcome the limited bio-
heparin cofactor II possesses a unique anionic sequence at its availability associated with this method of delivery.
N-terminal. In its unactivated state, this sequence forms an intramo- After entering the circulation, heparin binds to a variety of plasma
lecular bond with the positively charged glycosaminoglycan binding proteins other than antithrombin, which decreases the anticoagulant
site located on the body of heparin cofactor II. When heparin or activity of heparin. The levels of heparin-binding proteins vary
dermatan sulfate, a glycosaminoglycan that interacts only with between patients because some of these proteins are acute-phase
heparin cofactor II, binds to heparin cofactor II, this N-terminal reactants whose levels are elevated in ill patients, whereas others,
sequence is displaced, which enables its tethering to a positively such as high-molecular-weight multimers of von Willebrand factor,
charged domain on thrombin known as exosite 1. This tethering are released when platelets or endothelial cells are activated by throm-
interaction occurs only with thrombin and facilitates thrombin bin. Activated platelets also release platelet factor 4 (PF4), a highly
inhibition by heparin cofactor II (Fig. 149.6). cationic protein that binds heparin with high affinity. The large
Maximum catalysis of heparin cofactor II by heparin requires amount of PF4 found in the vicinity of platelet-rich arterial thrombi
heparin chains composed of at least 26 saccharide units, which cor- has the potential to locally neutralize the anticoagulant activity of
responds to a molecular weight of 7800. Longer heparin chains heparin.
activate heparin cofactor II to a greater extent than shorter chains Because the levels of heparin-binding proteins are so variable
because the longer chains are of sufficient length not only to bind to between patients, the anticoagulant response to fixed or weight-
heparin cofactor II but also to bind to thrombin to form a ternary adjusted doses of heparin is unpredictable. Consequently, coagulation
complex. Formation of this complex brings thrombin and heparin monitoring is essential to ensure that a therapeutic response is
cofactor II into close apposition. obtained when heparin is administered for treatment purposes.