Page 2441 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2441
Chapter 149 Antithrombotic Drugs 2179
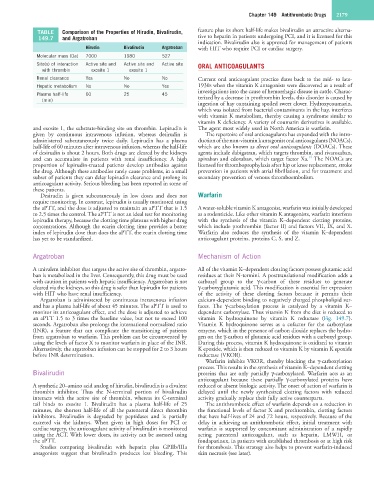
TABLE Comparison of the Properties of Hirudin, Bivalirudin, feature plus its short half-life makes bivalirudin an attractive alterna-
149.7 and Argatroban tive to heparin in patients undergoing PCI, and it is licensed for this
indication. Bivalirudin also is approved for management of patients
Hirudin Bivalirudin Argatroban with HIT who require PCI or cardiac surgery.
Molecular mass (Da) 7000 1980 527
Site(s) of interaction Active site and Active site and Active site ORAL ANTICOAGULANTS
with thrombin exosite 1 exosite 1
Renal clearance Yes No No Current oral anticoagulant practice dates back to the mid- to late-
Hepatic metabolism No No Yes 1930s when the vitamin K antagonists were discovered as a result of
investigations into the cause of hemorrhagic disease in cattle. Charac-
Plasma half-life 60 25 45 terized by a decrease in prothrombin levels, this disorder is caused by
(min)
ingestion of hay containing spoiled sweet clover. Hydroxycoumarin,
which was isolated from bacterial contaminants in the hay, interferes
with vitamin K metabolism, thereby causing a syndrome similar to
vitamin K deficiency. A variety of coumarin derivatives is available.
and exosite 1, the substrate-binding site on thrombin. Lepirudin is The agent most widely used in North America is warfarin.
given by continuous intravenous infusion, whereas desirudin is The repertoire of oral anticoagulants has expanded with the intro-
administered subcutaneously twice daily. Lepirudin has a plasma duction of the non-vitamin k antagonist oral anticoagulants (NOACs),
half-life of 60 minutes after intravenous infusion, whereas the half-life which are also known as direct oral anticoagulants (DOACs). These
of desirudin is about 2 hours. Both drugs are cleared by the kidneys agents include dabigatran, which targets thrombin, and rivaroxaban,
14
and can accumulate in patients with renal insufficiency. A high apixaban and edoxaban, which target factor Xa. The NOACs are
proportion of lepirudin-treated patients develop antibodies against licensed for thromboprophylaxis after hip or knee replacement, stroke
the drug. Although these antibodies rarely cause problems, in a small prevention in patients with atrial fibrillation, and for treatment and
subset of patients they can delay lepirudin clearance and prolong its secondary prevention of venous thromboembolism.
anticoagulant activity. Serious bleeding has been reported in some of
these patients.
Desirudin is given subcutaneously in low doses and does not Warfarin
require monitoring. In contrast, lepirudin is usually monitored using
the aPTT, and the dose is adjusted to maintain an aPTT that is 1.5 A water-soluble vitamin K antagonist, warfarin was initially developed
to 2.5 times the control. The aPTT is not an ideal test for monitoring as a rodenticide. Like other vitamin K antagonists, warfarin interferes
lepirudin therapy, because the clotting time plateaus with higher drug with the synthesis of the vitamin K–dependent clotting proteins,
concentrations. Although the ecarin clotting time provides a better which include prothrombin (factor II) and factors VII, IX, and X.
index of lepirudin dose than does the aPTT, the ecarin clotting time Warfarin also reduces the synthesis of the vitamin K–dependent
has yet to be standardized. anticoagulant proteins, proteins C, S, and Z.
Argatroban Mechanism of Action
A univalent inhibitor that targets the active site of thrombin, argatro- All of the vitamin K–dependent clotting factors possess glutamic acid
ban is metabolized in the liver. Consequently, this drug must be used residues at their N-termini. A posttranslational modification adds a
with caution in patients with hepatic insufficiency. Argatroban is not carboxyl group to the γ-carbon of these residues to generate
cleared via the kidneys, so this drug is safer than lepirudin for patients γ-carboxyglutamic acid. This modification is essential for expression
with HIT who have renal insufficiency. of the activity of these clotting factors because it permits their
Argatroban is administered by continuous intravenous infusion calcium-dependent binding to negatively charged phospholipid sur-
and has a plasma half-life of about 45 minutes. The aPTT is used to faces. The γ-carboxylation process is catalyzed by a vitamin K–
monitor its anticoagulant effect, and the dose is adjusted to achieve dependent carboxylase. Thus vitamin K from the diet is reduced to
an aPTT 1.5 to 3 times the baseline value, but not to exceed 100 vitamin K hydroquinone by vitamin K reductase (Fig. 149.7).
seconds. Argatroban also prolongs the international normalized ratio Vitamin K hydroquinone serves as a cofactor for the carboxylase
(INR), a feature that can complicate the transitioning of patients enzyme, which in the presence of carbon dioxide replaces the hydro-
from argatroban to warfarin. This problem can be circumvented by gen on the γ-carbon of glutamic acid residues with a carboxyl group.
using the levels of factor X to monitor warfarin in place of the INR. During this process, vitamin K hydroquinone is oxidized to vitamin
Alternatively, the argatroban infusion can be stopped for 2 to 3 hours K epoxide, which is then reduced to vitamin K by vitamin K epoxide
before INR determination. reductase (VKOR).
Warfarin inhibits VKOR, thereby blocking the γ-carboxylation
process. This results in the synthesis of vitamin K–dependent clotting
Bivalirudin proteins that are only partially γ-carboxylated. Warfarin acts as an
anticoagulant because these partially γ-carboxylated proteins have
A synthetic 20–amino acid analog of hirudin, bivalirudin is a divalent reduced or absent biologic activity. The onset of action of warfarin is
thrombin inhibitor. Thus the N-terminal portion of bivalirudin delayed until the newly synthesized clotting factors with reduced
interacts with the active site of thrombin, whereas its C-terminal activity gradually replace their fully active counterparts.
tail binds to exosite 1. Bivalirudin has a plasma half-life of 25 The antithrombotic effect of warfarin depends on a reduction in
minutes, the shortest half-life of all the parenteral direct thrombin the functional levels of factor X and prothrombin, clotting factors
inhibitors. Bivalirudin is degraded by peptidases and is partially that have half-lives of 24 and 72 hours, respectively. Because of the
excreted via the kidneys. When given in high doses for PCI or delay in achieving an antithrombotic effect, initial treatment with
cardiac surgery, the anticoagulant activity of bivalirudin is monitored warfarin is supported by concomitant administration of a rapidly
using the ACT. With lower doses, its activity can be assessed using acting parenteral anticoagulant, such as heparin, LMWH, or
the aPTT. fondaparinux, in patients with established thrombosis or at high risk
Studies comparing bivalirudin with heparin plus GPIIb/IIIa for thrombosis. This strategy also helps to prevent warfarin-induced
antagonists suggest that bivalirudin produces less bleeding. This skin necrosis (see later).