Page 271 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 271
222 Part III Immunologic Basis of Hematology
MHC II MHC I
5 3 6
4 MHC I
MHC II
4
5
MHC II MHC I
2 MHC I 4
3
1
MHC II Proteosome
ER
2
ER E3 ligase 1
Nucleus
Nucleus
A B
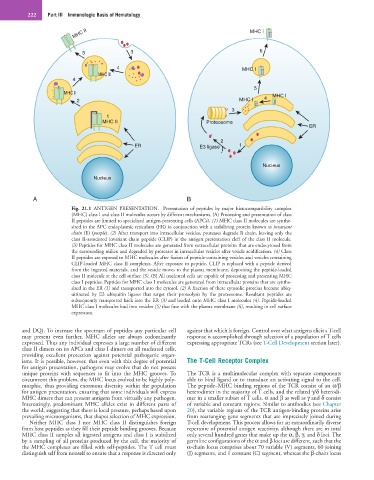
Fig. 21.1 ANTIGEN PRESENTATION. Presentation of peptides by major histocompatibility complex
(MHC) class I and class II molecules occurs by different mechanisms. (A) Processing and presentation of class
II peptides are limited to specialized antigen-presenting cells (APCs). (1) MHC class II molecules are synthe-
sized in the APC endoplasmic reticulum (ER) in conjunction with a stabilizing protein known as invariant
chain (Ii) (purple). (2) After transport into intracellular vesicles, proteases degrade Ii chain, leaving only the
class II-associated invariant chain peptide (CLIP) in the antigen presentation cleft of the class II molecule.
(3) Peptides for MHC class II molecules are generated from extracellular proteins that are endocytosed from
the surrounding milieu and degraded by proteases in intracellular vesicles after vesicle acidification. (4) Class
II peptides are exposed to MHC molecules after fusion of peptide-containing vesicles and vesicles containing
CLIP-loaded MHC class II complexes. After exposure to peptide, CLIP is replaced with a peptide derived
from the ingested materials, and the vesicle moves to the plasma membrane, depositing the peptide-loaded
class II molecule at the cell surface (5). (B) All nucleated cells are capable of processing and presenting MHC
class I peptides. Peptides for MHC class I molecules are generated from intracellular proteins that are synthe-
sized in the ER (1) and transported into the cytosol. (2) A fraction of these cytosolic proteins become ubiq-
uitinated by E3 ubiquitin ligases that target their proteolysis by the proteosome. Resultant peptides are
subsequently transported back into the ER (3) and loaded onto MHC class I molecules (4). Peptide-loaded
MHC class I molecules bud into vesicles (5) that fuse with the plasma membrane (6), resulting in cell surface
expression.
and DQ). To increase the spectrum of peptides any particular cell against that which is foreign. Control over what antigens elicit a T-cell
may present even further, MHC alleles are always codominantly response is accomplished through selection of a population of T cells
expressed. Thus any individual expresses a large number of different expressing appropriate TCRs (see T-Cell Development section later).
class II dimers on its APCs and class I dimers on all nucleated cells,
providing excellent protection against potential pathogenic organ-
isms. It is possible, however, that even with this degree of potential The T-Cell Receptor Complex
for antigen presentation, pathogens may evolve that do not possess
unique proteins with sequences to fit into the MHC grooves. To The TCR is a multimolecular complex with separate components
circumvent this problem, the MHC locus evolved to be highly poly- able to bind ligand or to transduce an activating signal to the cell.
morphic, thus providing enormous diversity within the population The peptide–MHC binding regions of the TCR consist of an α/β
for antigen presentation, ensuring that some individuals will express heterodimer in the majority of T cells, and the related γ/δ heterodi-
MHC dimers that can present antigens from virtually any pathogen. mer in a smaller subset of T cells. α and β as well as γ and δ consist
Interestingly, predominant MHC alleles exist in different parts of of variable and constant regions. Similar to antibodies (see Chapter
the world, suggesting that there is local pressure, perhaps based upon 20), the variable regions of the TCR antigen-binding proteins arise
prevailing microorganisms, that shapes selection of MHC expression. from rearranging gene segments that are imprecisely joined during
Neither MHC class I nor MHC class II distinguishes foreign T-cell development. This process allows for an extraordinarily diverse
from host peptides as they fill their peptide binding grooves. Because repertoire of potential antigen reactivity, although there are in total
MHC class II samples all ingested antigens and class I is stabilized only several hundred genes that make up the α, β, γ, and δ loci. The
by a sampling of all proteins produced by the cell, the majority of germline configurations of the α and β loci are different, such that the
the MHC complexes are filled with self-peptides. The T cell must α-chain locus comprises about 70 variable (V) segments, 60 joining
distinguish self from nonself to ensure that a response is directed only (J) segments, and 1 constant (C) segment, whereas the β-chain locus