Page 276 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 276
Chapter 21 T-Cell Immunity 227
killer (NK) T cells, a subtype of lymphocytes that has characteristics interactions between Notch receptors on the developing T cells and
of both T and NK cells (see Chapter 22), are also generated in the specific Notch ligands collaborate with signaling through the IL-7
thymus. It has become clear recently that additional small popula- cytokine receptor to regulate lineage commitment and developmental
tions of T cells possessing unique characteristics are also produced in progression.
the thymus. This chapter focuses primarily on αβ T cells and touches During the DN3 stage, rearrangement of TCR-γ, TCR-δ, and
briefly on γδ T-cell development. TCR-β loci occurs with maximal efficiency, and initial expression
of the TCR proteins these genes encode occurs. From this time
onward in T-cell development, the proliferation and survival of the
Early T-Cell Development developing thymocytes depend on TCR signals. Two key checkpoints
must be passed for full T-cell development to occur. First, upon
Identifying T-cell progenitors is an area of intense investigation productive rearrangement of the TCR-β locus, the TCR-β protein
because developing tools to manipulate these cells has great therapeu- forms a “pre-TCR” complex with an invariant cytosolic protein des-
tic potential for increasing the speed at which T-cell repopulation may ignated pre-Tα. This complex engages the TCR signaling machinery,
occur following hematopoietic stem cell transplant. A population including the PTKs Lck, Fyn, and Syk (a ZAP-70-related PTK) and
of bone marrow–derived thymic settling progenitors (TSPs) that the adapters SLP-76 and LAT, to initiate the TCR signaling cascade.
can give rise to mature T-cell populations has been identified. As The resultant biochemical second messengers suppress rearrangement
these cells enter the thymus at the corticomedullary junction, they of the other β allele resulting in “allelic exclusion,” or silencing of the
develop into double-negative (DN) T cells, characterized by lack of nonrearranged allele, to ensure each T cell expresses only one TCR
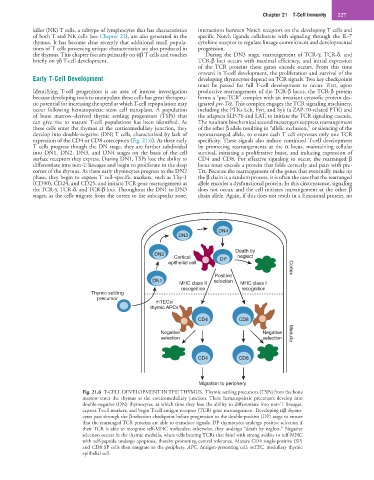
expression of the CD4 or CD8 coreceptors (Fig. 21.6). As these early specificity. These signals also induce continued T-cell development
T cells progress though the DN stage, they are further subdivided by promoting rearrangements at the α locus, maintaining cellular
into DN1, DN2, DN3, and DN4 stages on the basis of the cell survival, initiating a proliferative burst, and inducing expression of
surface receptors they express. During DN1, TSPs lose the ability to CD4 and CD8. For effective signaling to occur, the rearranged β
differentiate into non-T lineages and begin to proliferate in the deep locus must encode a protein that folds correctly and pairs with pre-
cortex of the thymus. As these early thymocytes progress to the DN2 Tα. Because the rearrangement of the genes that eventually make up
phase, they begin to express T cell–specific markers, such as Thy-1 the β chain is a random process, it is often the case that the rearranged
(CD90), CD24, and CD25, and initiate TCR gene rearrangement at allele encodes a dysfunctional protein. In this circumstance, signaling
the TCR-γ, TCR-δ, and TCR-β loci. Throughout the DN1 to DN3 does not occur, and the cell initiates rearrangement at the other β
stages, as the cells migrate from the cortex to the subcapsular zone, chain allele. Again, if this does not result in a functional protein, no
DN4
DN3
Death by
DN2
Cortical DP neglect
epithelial cell Cortex
Positive
DN1 selection
MHC class II MHC class I
recognition recognition
Thymic settling
precursor
mTECs/
thymic APCs
CD4 CD8
Negative Negative Medulla
selection selection
CD4 CD8
Migration to periphery
Fig. 21.6 T-CELL DEVELOPMENT IN THE THYMUS. Thymic settling precursors (TSPs) from the bone
marrow enter the thymus at the corticomedullary junction. These hematopoietic precursors develop into
double-negative (DN) thymocytes, at which time they lose the ability to differentiate into non-T lineages,
express T-cell markers, and begin T-cell antigen receptor (TCR) gene rearrangement. Developing αβ thymo-
cytes pass through the β-selection checkpoint before progression to the double-positive (DP) stage to ensure
that the rearranged TCR proteins are able to transduce signals. DP thymocytes undergo positive selection if
their TCR is able to recognize self-MHC molecules; otherwise, they undergo “death by neglect.” Negative
selection occurs in the thymic medulla, when cells bearing TCRs that bind with strong avidity to self-MHC
with self-peptide undergo apoptosis, thereby promoting central tolerance. Mature CD4 single-positive (SP)
and CD8 SP cells then emigrate to the periphery. APC, Antigen-presenting cell; mTEC, medullary thymic
epithelial cell.