Page 336 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 336
278 Part III Immunologic Basis of Hematology
SS SS SS SS SS SS SS SS
SS SS SS SS
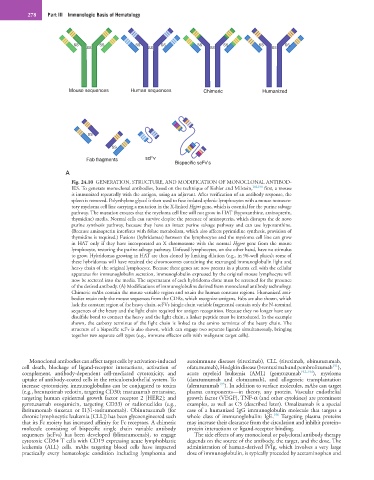
Mouse sequences Human sequences Chimeric Humanized
SS
SS
Fab fragments scFv
Bispecific scFv’s
A
Fig. 24.10 GENERATION, STRUCTURE, AND MODIFICATION OF MONOCLONAL ANTIBOD-
IES. To generate monoclonal antibodies, based on the technique of Kohler and Milstein, 182,190 first, a mouse
is immunized repeatedly with the antigen, using an adjuvant. After verification of an antibody response, the
spleen is removed. Polyethylene glycol is then used to fuse isolated splenic lymphocytes with a mouse nonsecre-
tory myeloma cell line carrying a mutation in the X-linked Hgprt gene, which is essential for the purine salvage
pathway. The mutation ensures that the myeloma cell line will not grow in HAT (hypoxanthine, aminopterin,
thymidine) media. Normal cells can survive despite the presence of aminopterin, which disrupts the de novo
purine synthesis pathway, because they have an intact purine salvage pathway and can use hypoxanthine.
(Because aminopterin interferes with folate metabolism, which also affects pyrimidine synthesis, provision of
thymidine is required.) Fusions (hybridomas) between the lymphocytes and the myeloma cell line can grow
in HAT only if they have incorporated an X chromosome with the normal Hgprt gene from the mouse
lymphocyte, restoring the purine salvage pathway. Unfused lymphocytes, on the other hand, have no stimulus
to grow. Hybridomas growing in HAT are then cloned by limiting dilution (e.g., in 96-well plates); some of
these hybridomas will have retained the chromosomes containing the rearranged immunoglobulin light and
heavy chain of the original lymphocyte. Because these genes are now present in a plasma cell with the cellular
apparatus for immunoglobulin secretion, immunoglobulin expressed by the original mouse lymphocyte will
now be secreted into the media. The supernatant of each hybridoma clone must be screened for the presence
of the desired antibody. (A) Modifications of immunoglobulins derived from monoclonal antibody technology.
Chimeric mAbs contain the mouse variable region and retain the human constant regions. Humanized anti-
bodies retain only the mouse sequences from the CDRs, which recognize antigens. Fabs are also shown, which
lack the constant region of the heavy chain. scFVs (single chain variable fragments) contain only the N-terminal
sequences of the heavy and the light chain required for antigen recognition. Because they no longer have any
disulfide bond to connect the heavy and the light chain, a linker peptide must be introduced. In the example
shown, the carboxy terminus of the light chain is linked to the amino terminus of the heavy chain. The
structure of a bispecific scFv is also shown, which can engage two separate ligands simultaneously, bringing
together two separate cell types (e.g., immune effector cells with malignant target cells).
Monoclonal antibodies can affect target cells by activation-induced autoimmune diseases (rituximab), CLL (rituximab, obinutuzumab,
191
cell death, blockage of ligand-receptor interactions, activation of ofatumumab), Hodgkin disease (brentuximab and pembrolizumab ),
complement, antibody-dependent cell-mediated cytotoxicity, and acute myeloid leukemia (AML) (gemtuzumab 192–194 ), myeloma
uptake of antibody-coated cells in the reticuloendothelial system. To (daratumumab and elotuzumab), and allogeneic transplantation
195
increase cytotoxicity, immunoglobulins can be conjugated to toxins (alemtuzumab ). In addition to surface molecules, mAbs can target
(e.g., brentuximab vedotin, targeting CD30; trastuzumab emtansine, plasma components—in theory, any protein. Vascular endothelial
targeting human epidermal growth factor receptor 2 [HER2]; and growth factor (VEGF), TNF-α (and other cytokines) are prominent
gemtuzumab ozogamicin, targeting CD33) or radionuclides (e.g., examples, as well as C5 (described later). Omalizumab is a special
ibritumomab tiuxetan or I131-tositumomab). Obinutuzumab (for case of a humanized IgG immunoglobulin molecule that targets a
196
chronic lymphocytic leukemia [CLL]) has been glycoengineered such whole class of immunoglobulin: IgE. Targeting plasma proteins
that its Fc moiety has increased affinity for Fc receptors. A chimeric may increase their clearance from the circulation and inhibit protein–
molecule consisting of bispecific single chain variable antibody protein interactions or ligand-receptor binding.
sequences (scFvs) has been developed (blinatumomab), to engage The side effects of any monoclonal or polyclonal antibody therapy
cytotoxic CD3+ T cells with CD19 expressing acute lymphoblastic depends on the source of the antibody, the target, and the dose. The
leukemia (ALL) cells. mAbs targeting blood cells have impacted administration of human-derived IVIg, which involves a very large
practically every hematologic condition including lymphoma and dose of immunoglobulin, is typically preceded by acetaminophen and