Page 564 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 564
Chapter 36 Disorders of Iron Homeostasis 479
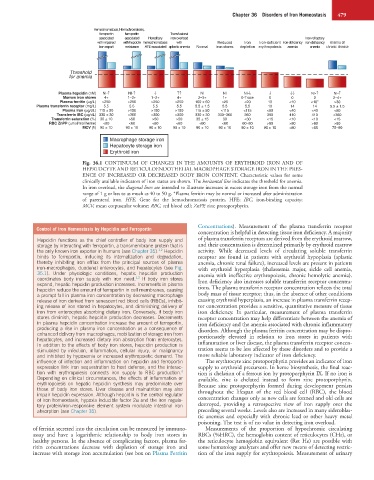
Hemochromatosis, Hemochromatosis,
ferroportin ferroportin Transfusional
associated associated Hereditary iron overload Iron-refractory
with impaired with hepcidin hemochromatosis with Reduced Iron Iron-deficient Iron-deficiency iron-deficiency Anemia of
iron export resistance HFE-associated aplastic anemia Normal iron stores depletion erythropoiesis anemia anemia chronic disease
Threshold
for anemia
Plasma hepcidin (nM) NI-↑ NI-↑ ↓ ↑↑ NI NI NI-↓ ↓ ↓↓ NI-↑ NI-↑
Marrow iron stores 4+ 1–2+ 1–2+ 4+ 2–3+ 1+ 0-Trace 0 0 0 2–4+
Plasma ferritin (µg/L) >250 >250 >250 >250 100 ± 60 <25 <20 10 <10 <10* >30
Plasma transferrin receptor (mg/L) 5.5 5.5 5.5 5.5 5.5 ± 1.5 5.5 5.5 10 14 14 5.5 ± 1.5
Plasma iron (µg/dL) 115 ± 50 >150 >150 >150 115 ± 50 <115 <115 <60 <40 <40 <60
Transferrin IBC (µg/dL) 330 ± 30 <300 <300 <300 330 ± 30 330–360 360 390 410 410 <360
Transferrin saturation (%) 30 ± 10 >50 >50 >50 35 ± 15 30 <30 <15 <10 <10 <15
RBC ZnPP (µmol/mol heme) <60 <60 <60 <60 <60 <60 60–80 >80 >80 >80 >60
MCV (fl) 90 ± 10 90 ± 10 90 ± 10 90 ± 10 90 ± 10 90 ± 10 90 ± 10 90 ± 10 <80 <65 75–90
Macrophage storage iron
Hepatocyte storage iron
Erythroid iron
Fig. 36.1 CONTINUUM OF CHANGES IN THE AMOUNTS OF ERYTHROID IRON AND OF
HEPATOCYTE AND RETICULOENDOTHELIAL MACROPHAGE STORAGE IRON IN THE PRES-
ENCE OF INCREASED OR DECREASED BODY IRON CONTENT. Characteristic values for some
clinically available indicators of iron status are shown. The horizontal line indicates the threshold for anemia.
In iron overload, the diagonal lines are intended to illustrate increases in excess storage iron from the normal
range of 1 g or less to as much as 40 to 50 g. *Plasma ferritin may be normal or increased after administration
of parenteral iron. HFE, Gene for the hemochromatosis protein, HFE; IBC, iron-binding capacity;
MCV, mean corpuscular volume; RBC, red blood cell; ZnPP, zinc protoporphyrin.
Concentrations). Measurement of the plasma transferrin receptor
Control of Iron Homeostasis by Hepcidin and Ferroportin
concentration is helpful in detecting tissue iron deficiency. A majority
Hepcidin functions as the chief controller of body iron supply and of plasma transferrin receptors are derived from the erythroid marrow,
storage by interacting with ferroportin, a transmembrane protein that is and their concentration is determined primarily by erythroid marrow
the only known iron exporter in humans (see Chapter 35). Hepcidin activity. While decreased levels of circulating soluble transferrin
1,2
binds to ferroportin, inducing its internalization and degradation, receptor are found in patients with erythroid hypoplasia (aplastic
thereby inhibiting iron efflux from the principal sources of plasma anemia, chronic renal failure), increased levels are present in patients
iron-macrophages, duodenal enterocytes, and hepatocytes (see Fig. with erythroid hyperplasia (thalassemia major, sickle cell anemia,
35.1). Under physiologic conditions, hepatic hepcidin production anemia with ineffective erythropoiesis, chronic hemolytic anemia).
coordinates body iron supply with iron need. If body iron stores Iron deficiency also increases soluble transferrin receptor concentra-
1,2
expand, hepatic hepcidin production increases. Increments in plasma
hepcidin reduce the amount of ferroportin in cell membranes, causing tions. The plasma transferrin receptor concentration reflects the total
a prompt fall in plasma iron concentration by decreasing macrophage body mass of tissue receptor; thus, in the absence of other conditions
release of iron derived from senescent red blood cells (RBCs), inhibit- causing erythroid hyperplasia, an increase in plasma transferrin recep-
ing release of iron stored in hepatocytes, and diminishing delivery of tor concentration provides a sensitive, quantitative measure of tissue
iron from enterocytes absorbing dietary iron. Conversely, if body iron iron deficiency. In particular, measurement of plasma transferrin
stores diminish, hepatic hepcidin production decreases. Decrements receptor concentration may help differentiate between the anemia of
in plasma hepcidin concentration increase the amount of ferroportin, iron deficiency and the anemia associated with chronic inflammatory
producing a rise in plasma iron concentration as a consequence of disorders. Although the plasma ferritin concentration may be dispro-
enhanced delivery from macrophages, mobilization of storage iron from portionately elevated in relation to iron stores in patients with
hepatocytes, and increased dietary iron absorption from enterocytes.
In addition to the effects of body iron stores, hepcidin production is inflammation or liver disease, the plasma transferrin receptor concen-
stimulated by infection, inflammation, cellular injury, or malignancy tration seems to be less affected by these disorders and to provide a
and inhibited by hypoxemia or increased erythropoietic demand. The more reliable laboratory indicator of iron deficiency.
influence of infection and inflammation on hepcidin and ferroportin The erythrocyte zinc protoporphyrin provides an indicator of iron
expression link iron sequestration to host defense, and the interac- supply to erythroid precursors. In heme biosynthesis, the final reac-
tion with erythropoiesis connects iron supply to RBC production. tion is chelation of a ferrous ion by protoporphyrin IX. If no iron is
3
Depending on clinical circumstances, the effects of inflammation or available, zinc is chelated instead to form zinc protoporphyrin.
erythropoiesis on hepatic hepcidin synthesis may predominate over Because zinc protoporphyrin formed during development persists
those of body iron stores. Liver disease and malnutrition may also throughout the lifespan of the red blood cell (RBC), the blood
impair hepcidin expression. Although hepcidin is the central regulator
of iron homeostasis, hypoxia inducible factor 2α and the iron regula- concentration changes only as new cells are formed and old cells are
tory protein/iron-responsive element system modulate intestinal iron destroyed, providing a retrospective view of iron supply over the
absorption (see Chapter 35). preceding several weeks. Levels also are increased in many sideroblas-
tic anemias and especially with chronic lead or other heavy metal
poisoning. The test is of no value in detecting iron overload.
of ferritin secreted into the circulation can be measured by immuno- Measurements of the proportion of hypochromic circulating
assay and have a logarithmic relationship to body iron stores in RBCs (%HRC), the hemoglobin content of reticulocytes (CHr), or
healthy persons. In the absence of complicating factors, plasma fer- the reticulocyte hemoglobin equivalent (Ret He) are possible with
ritin concentrations decrease with depletion of storage iron and some hematology analyzers and offer new means of detecting restric-
increase with storage iron accumulation (see box on Plasma Ferritin tion of the iron supply for erythropoiesis. Measurement of urinary