Page 561 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 561
476 Part V Red Blood Cells
iron-regulated BMP-SMAD pathway. Still other signal transduction INTESTINAL IRON ABSORPTION
pathways seem likely to be involved in regulating hepatic hepcidin
production in response to increased erythropoietic requirements. Because humans are unable to excrete excess iron, iron balance is
Transferrin receptor 1 expression on erythroid precursors has been physiologically maintained by the control of iron absorption in the
proposed as a proximal mediator of erythropoietic control of hepcidin proximal portion of the duodenum. Iron overload develops if regula-
expression, acting through a yet to be identified soluble regulator tion of iron balance is bypassed by parenteral injections of iron or
produced by the erythroid marrow. 27 transfusion. Normally, only about 1 to 1.5 mg of iron of the
10 to 20 mg in the adult diet is absorbed to balance obligatory losses.
Inflammatory and Endoplasmic Reticulum Both nonheme iron and heme iron enter through the microvillous
brush border at the apical (luminal) surface of the intestinal entero-
Stress–Related Regulation of Hepcidin Expression cytes (Fig. 35.8). Nonheme dietary iron is predominantly ferric (Fe )
3+
2+
and, before absorption, is converted to ferrous (Fe ) iron either by
Inflammation increases plasma hepcidin, resulting in retention of the reducing action of other dietary constituents or by the action of
iron within macrophages, reduced iron absorption, and hypoferre- brush border ferrireductases, such as membrane-associated duodenal
28
mia. The inflammatory cytokine interleukin-6 (IL-6) induces cytochrome B (DCYTB) and likely others. The ferrous iron is then
hepcidin expression (see Fig. 35.7). IL-6 activates the Janus kinase– absorbed through DMT1, the same ferrous iron transporter that
signal transducer and activator of transcription (JAK-STAT) signaling provides an exit for iron from the endosome (see earlier). The exact
pathway, stimulating hepcidin production through STAT3 interac- means by which heme iron is absorbed are still uncertain, but, when
tions with a STAT3-binding element in the hepcidin promoter. inside the enterocyte, inducible heme oxygenase 1 releases the iron
Other cytokines and the BMP6-HJV-SMAD pathway may also be from protoporphyrin, apparently into a common pathway with
4
involved. In addition, the acute inflammatory response is linked to
ER stress (see Fig. 35.7), resulting from accumulation of unfolded or
misfolded proteins with disruption of ER homeostasis. Hepcidin
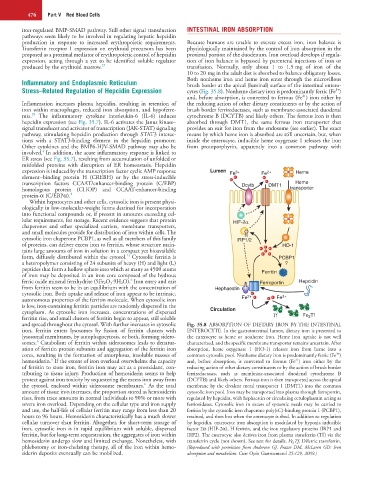
expression is induced by the transcription factor cyclic AMP response Lumen Fe 3+ Fe 2+ Heme
element−binding protein H (CREBH) or by the stress-inducible
transcription factors CCAAT/enhancer-binding protein (C/EBP) Dcytb DMT1 Heme
homologous protein (CHOP) and CCAAT-enhancer-binding transporter
protein-α (C/EBPα). 4
Within hepatocytes and other cells, cytosolic iron is present physi-
ologically in low-molecular-weight forms destined for incorporation
into functional compounds or, if present in amounts exceeding cel-
lular requirements, for storage. Recent evidence suggests that protein HIF-2α
chaperones and other specialized carriers, membrane transporters,
and small molecules provide for distribution of iron within cells. The
cytosolic iron chaperone PCBP1, as well as all members of this family IRP1/2
of proteins, can deliver excess iron to ferritin, whose structure main- HO-1
tains large amounts of iron in solution in a compact yet bioavailable
13
form, diffusely distributed within the cytosol. Cytosolic ferritin is PCBP1
a heteropolymer consisting of 24 subunits of heavy (H) and light (L) ?
peptides that form a hollow sphere into which as many as 4500 atoms Ferritin
of iron may be deposited in an iron core composed of the hydrous
6
ferric oxide mineral ferrihydrite (5Fe 2 O 3 ⋅9H 2 O). Iron entry and exit Ferroportin Hepcidin
from ferritin seem to be in an equilibrium with the concentration of Hephaestin
cytosolic iron. Both uptake and release of iron appear to be intrinsic, 2+ 3+
autonomous properties of the ferritin molecule. When cytosolic iron Fe Fe
is low, iron-containing ferritin particles are randomly dispersed in the Circulation
cytoplasm. As cytosolic iron increases, concentrations of dispersed Fe 2 Tf
ferritin rise, and small clusters of ferritin begin to appear, still soluble Tf
and spread throughout the cytosol. With further increases in cytosolic Fig. 35.8 ABSORPTION OF DIETARY IRON BY THE INTESTINAL
iron, ferritin enters lysosomes by fusion of ferritin clusters with ENTEROCYTE. In the gastrointestinal lumen, dietary iron is presented to
lysosomal membranes, by autophagocytosis, or both, forming sidero- the enterocyte as heme or nonheme iron. Heme iron uptake is not well
6
somes. Catabolism of ferritin within siderosomes leads to denatur- characterized, and the specific membrane transporter remains uncertain. After
ation of ferritin protein subunits and aggregation of the ferritin iron absorption, heme oxygenase 1 (HO-1) releases iron from heme into a
3+
cores, resulting in the formation of amorphous, insoluble masses of common cytosolic pool. Nonheme dietary iron is predominantly ferric (Fe )
6
2+
hemosiderin. If the extent of iron overload overwhelms the capacity and, before absorption, is converted to ferrous (Fe ) iron either by the
of ferritin to store iron, ferritin iron may act as a prooxidant, con- reducing action of other dietary constituents or by the action of brush border
tributing to tissue injury. Production of hemosiderin seems to help ferrireductases, such as membrane-associated duodenal cytochrome B
protect against iron toxicity by sequestering the excess iron away from (DCYTB) and likely others. Ferrous iron is then transported across the apical
6
the cytosol, enclosed within siderosome membranes. As the total membrane by the divalent metal transporter 1 (DMT1) into the common
amount of tissue iron increases, the proportion stored as hemosiderin cytosolic iron pool. Iron may be transported into plasma through ferroportin,
rises, from trace amounts in normal individuals to 90% or more with regulated by hepcidin, with hephaestin or circulating ceruloplasmin acting as
severe iron overload. Depending on the cellular type and iron supply ferrioxidases. Cytosolic iron in excess of systemic needs may be carried to
and use, the half-life of cellular ferritin may range from less than 20 ferritin by the cytosolic iron chaperone poly(rC)-binding protein 1 (PCBP1),
hours to 96 hours. Hemosiderin characteristically has a much slower retained, and then lost when the enterocyte is shed. In addition to regulation
cellular turnover than ferritin. Altogether, for short-term storage of by hepcidin, enterocyte iron absorption is modulated by hypoxia inducible
iron, cytosolic iron is in rapid equilibrium with soluble, dispersed factor 2α (HIF-2α), H ferritin, and the iron regulatory proteins (IRP1 and
ferritin, but for long-term sequestration, the aggregates of iron within IRP2). The enterocyte also derives iron from plasma transferrin (Tf) via the
hemosiderin undergo slow and limited exchange. Nonetheless, with transferrin cycle (not shown). See text for details. Fe 2 Tf, Diferric transferrin.
phlebotomy or iron-chelating therapy, all of the iron within hemo- (Reproduced with permission from Anderson GJ, Frazer DM, McLaren GD: Iron
siderin deposits eventually can be mobilized. absorption and metabolism. Curr Opin Gastroenterol 25:129, 2009.)