Page 560 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 560
Chapter 35 Pathophysiology of Iron Homeostasis 475
Erythropoiesis Iron status
Circulating iron Iron stores
RBCs
Diferric transferrin
Hemojuvelin
pO 2
Neogenin
− BMP-6 Inflammation
Soluble
Erythropoietin hemojuvelin Interleukin-6
Erythroferrone ?
Interleukin-6
? HFE receptor
TFR2
TFR1 ? BMPR Matriptase-2
− ERK I-II Soluble
Hepatocellular SMAD Furin hemojuvelin
membrane
? + + JAK-STAT
+ HIF
Oxygen
Nucleus tension +
? ?
?
Promoter
HAMP
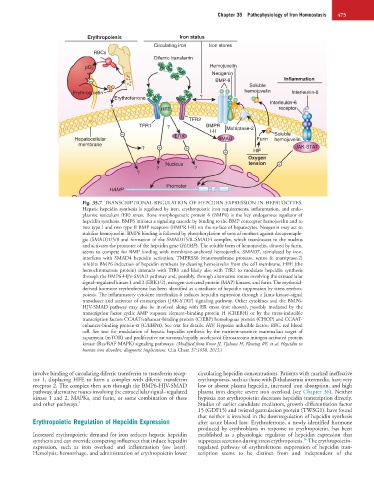
Fig. 35.7 TRANSCRIPTIONAL REGULATION OF HEPCIDIN EXPRESSION IN HEPATOCYTES.
Hepatic hepcidin synthesis is regulated by iron, erythropoietic iron requirements, inflammation, and endo-
plasmic reticulum (ER) stress. Bone morphogenetic protein 6 (BMP6) is the key endogenous regulator of
hepcidin synthesis. BMP6 initiates a signaling cascade by binding to the BMP coreceptor hemojuvelin and to
two type I and two type II BMP receptors (BMPR I-II) on the surface of hepatocytes. Neogenin may act to
stabilize hemojuvelin. BMP6 binding is followed by phosphorylation of sons of mothers against decapentaple-
gic (SMAD)1/5/8 and formation of the SMAD1/5/8–SMAD4 complex, which translocates to the nucleus
and activates the promoter of the hepcidin gene (HAMP). The soluble form of hemojuvelin, cleaved by furin,
seems to compete for BMP binding with membrane-anchored hemojuvelin. SMAD7, stimulated by iron,
interferes with SMAD4 hepcidin activation. TMPRSS6 (transmembrane protease, serine 6; matriptase-2)
inhibits BMP6 induction of hepcidin synthesis by cleaving hemojuvelin from the cell membrane. HFE (the
hemochromatosis protein) interacts with TfR1 and likely also with TfR2 to modulate hepcidin synthesis
through the BMP6-HJV-SMAD pathway and, possibly, through alternative routes involving the extracellular
signal–regulated kinase 1 and 2 (ERK1/2), mitogen-activated protein (MAP) kinases, and furin. The erythroid-
derived hormone erythroferrone has been identified as a mediator of hepcidin suppression by stress erythro-
poiesis. The inflammatory cytokine interleukin-6 induces hepcidin expression through a Janus kinase–signal
transducer and activator of transcription (JAK-STAT) signaling pathway. Other cytokines and the BMP6-
HJV-SMAD pathway may also be involved along with ER stress (not shown), possibly mediated by the
transcription factor cyclic AMP response element–binding protein H (CREBH) or by the stress-inducible
transcription factors CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) and CCAAT-
enhancer-binding protein-α (C/EBPα). See text for details. HIF, Hypoxia inducible factor; RBC, red blood
cell. See text for modulation of hepatic hepcidin synthesis by the nutrient-sensitive mammalian target of
rapamycin (mTOR) and proliferative rat sarcoma/rapidly accelerated fibrosarcoma mitogen-activated protein
kinase (Ras/RAF MAPK) signaling pathways. (Modified from Kroot JJ, Tjalsma H, Fleming RE, et al: Hepcidin in
human iron disorders: diagnostic implications. Clin Chem 57:1650, 2011.)
involve binding of circulating diferric transferrin to transferrin recep- circulating hepcidin concentrations. Patients with marked ineffective
tor 1, displacing HFE to form a complex with diferric transferrin erythropoiesis, such as those with β-thalassemia intermedia, have very
receptor 2. The complex then acts through the BMP6-HJV-SMAD low or absent plasma hepcidin, increased iron absorption, and high
pathway, alternative routes involving the extracellular signal−regulated plasma iron despite severe iron overload (see Chapter 36). Neither
kinase 1 and 2, MAPKs, and furin, or some combination of these hypoxia nor erythropoietin decreases hepcidin transcription directly.
and other pathways. 4 Studies of earlier candidate mediators, growth differentiation factor
15 (GDF15) and twisted gastrulation protein (TWSG1), have found
that neither is involved in the downregulation of hepcidin synthesis
Erythropoietic Regulation of Hepcidin Expression after acute blood loss. Erythroferrone, a newly identified hormone
produced by erythroblasts in response to erythropoietin, has been
Increased erythropoietic demand for iron reduces hepatic hepcidin established as a physiologic regulator of hepcidin expression that
26
synthesis and can override competing influences that induce hepcidin suppresses secretion during stress erythropoiesis. The erythropoietin-
expression, such as iron overload and inflammation (see later). regulated pathway of erythroferrone suppression of hepcidin tran-
Hemolysis, hemorrhage, and administration of erythropoietin lower scription seems to be distinct from and independent of the