Page 559 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 559
474 Part V Red Blood Cells
Tf
Heme–hemopexin
Fe 2 Tf Mitochondria
CD91
Heme
TFR1
TFR1
HO-1
3+
Non–transferrin- Fe Cytoplasmic
bound iron iron pool Bile
(NTBI) STEAP3 Fe 2+ secretion
Ferrireductase Lysosomes
Fe 3+
DMT1
Fe 2+ Ferritin
? DMT1 Endosomes
? ZIP14
Ferroportin
LRP,
Ceruloplasmin RHL-1
Fe 3+ Tf
Fe 2+ Lactoferrin
Fe 2 Tf
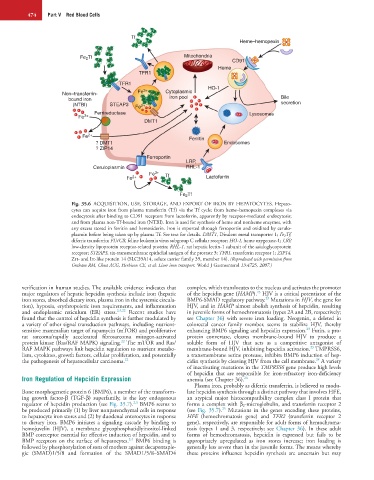
Fig. 35.6 ACQUISITION, USE, STORAGE, AND EXPORT OF IRON BY HEPATOCYTES. Hepato-
cytes can acquire iron from plasma transferrin (Tf) via the Tf cycle; from heme–hemopexin complexes via
endocytosis after binding to CD91 receptors from lactoferrin, apparently by receptor-mediated endocytosis;
and from plasma non-Tf-bound iron (NTBI). Iron is used for synthesis of heme and nonheme enzymes, with
any excess stored in ferritin and hemosiderin. Iron is exported through ferroportin and oxidized by cerulo-
plasmin before being taken up by plasma Tf. See text for details. DMT1, Divalent metal transporter 1; Fe 2Tf,
diferric transferrin; FLVCR, feline leukemia virus subgroup C cellular receptor; HO-1, heme oxygenase-1; LRP,
low-density lipoprotein receptor-related protein; RHL-1, rat hepatic lectin-1 subunit of the asialoglycoprotein
receptor; STEAP3, six-transmembrane epithelial antigen of the prostate 3; TFR1, transferrin receptor 1; ZIP14,
Zrt- and Irt-like protein 14 (SLC39A14, solute carrier family 39, member 14). (Reproduced with permission from
Graham RM, Chua ACG, Herbison CE, et al: Liver iron transport. World J Gastroenterol 13:4725, 2007.)
verification in human studies. The available evidence indicates that complex, which translocates to the nucleus and activates the promoter
23
major regulators of hepatic hepcidin synthesis include iron (hepatic of the hepcidin gene (HAMP). HJV is a critical potentiator of the
23
iron stores, absorbed dietary iron, plasma iron in the systemic circula- BMP6-SMAD regulatory pathway. Mutations in HJV, the gene for
tion), hypoxia, erythropoietic iron requirements, and inflammation HJV, and in HAMP almost abolish synthesis of hepcidin, resulting
and endoplasmic reticulum (ER) stress. 2,4,22 Recent studies have in juvenile forms of hemochromatosis (types 2A and 2B, respectively;
found that the control of hepcidin synthesis is further modulated by see Chapter 36) with severe iron loading. Neogenin, a deleted in
a variety of other signal transduction pathways, including nutrient- colorectal cancer family member, seems to stabilize HJV, thereby
23
sensitive mammalian target of rapamycin (mTOR) and proliferative enhancing BMP6 signaling and hepcidin expression. Furin, a pro-
rat sarcoma/rapidly accelerated fibrosarcoma mitogen-activated protein convertase, cleaves membrane-bound HJV to produce a
22
protein kinase (Ras/RAF MAPK) signaling. The mTOR and Ras/ soluble form of HJV that acts as a competitive antagonist of
23
RAF MAPK pathways link hepcidin regulation to nutrient metabo- membrane-bound HJV, inhibiting hepcidin activation. TMPRSS6,
lism, cytokines, growth factors, cellular proliferation, and potentially a transmembrane serine protease, inhibits BMP6 induction of hep-
24
the pathogenesis of hepatocellular carcinoma. 22 cidin synthesis by cleaving HJV from the cell membrane. A variety
of inactivating mutations in the TMPRSS6 gene produce high levels
of hepcidin that are responsible for iron-refractory iron-deficiency
Iron Regulation of Hepcidin Expression anemia (see Chapter 36). 24
Plasma iron, probably as diferric transferrin, is believed to modu-
Bone morphogenetic protein 6 (BMP6), a member of the transform- late hepcidin synthesis through a distinct pathway that involves HFE,
ing growth factor-β (TGF-β) superfamily, is the key endogenous an atypical major histocompatibility complex class I protein that
2,4
regulator of hepcidin production (see Fig. 35.7). BMP6 seems to forms a complex with β 2-microglobulin, and transferrin receptor 2
25
be produced primarily (1) by liver nonparenchymal cells in response (see Fig. 35.7). Mutations in the genes encoding these proteins,
to hepatocyte iron stores and (2) by duodenal enterocytes in response HFE (hemochromatosis gene) and TFR2 (transferrin receptor 2
to dietary iron. BMP6 initiates a signaling cascade by binding to gene), respectively, are responsible for adult forms of hemochroma-
hemojuvelin (HJV), a membrane glycophosphatidlyinositol-linked tosis (types 1 and 3, respectively; see Chapter 36). In these adult
BMP coreceptor essential for effective induction of hepcidin, and to forms of hemochromatosis, hepcidin is expressed but fails to be
2,4
BMP receptors on the surface of hepatocytes. BMP6 binding is appropriately upregulated as iron stores increase; iron loading is
followed by phosphorylation of sons of mothers against decapentaple- generally less severe than in the juvenile forms. The means whereby
gic (SMAD)1/5/8 and formation of the SMAD1/5/8–SMAD4 these proteins influence hepcidin synthesis are uncertain but may