Page 1101 - Williams Hematology ( PDFDrive )
P. 1101
1076 Part VIII: Monocytes and Macrophages Chapter 68: Production, Distribution, and Activation of Monocytes and Macrophages 1077
macrophages in marrow erythropoietic islands is poorly understood, as development, both in hematopoiesis and in extravascular tissues, and
is the considerable role of macrophages in iron and heme metabolism. much remains to be learned regarding their properties in the fetus.
Macrophages interact with cells in numerous ways; however, during
erythropoiesis a special phagocytic process allows for the removal of Growth, Differentiation, and Turnover
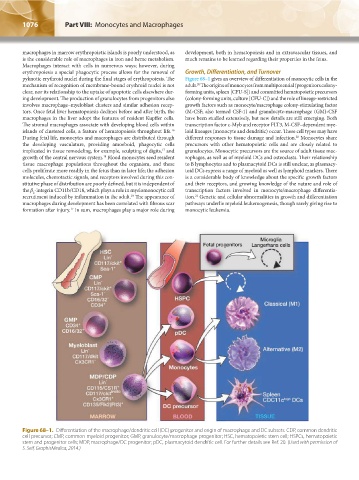
pyknotic erythroid nuclei during the final stages of erythropoiesis. The Figure 68–1 gives an overview of differentiation of monocytic cells in the
mechanism of recognition of membrane-bound erythroid nuclei is not adult. The origins of monocytes from multipotential (progenitors colony-
20
clear, nor its relationship to the uptake of apoptotic cells elsewhere dur- forming units, spleen [CFU-S]) and committed hematopoietic precursors
ing development. The production of granulocytes from progenitors also (colony-forming units, culture [CFU-C]) and the role of lineage-restricted
involves macrophage–myeloblast clusters and similar adhesion recep- growth factors such as monocyte/macrophage colony-stimulating factor
tors. Once fetal liver hematopoiesis declines before and after birth, the (M-CSF; also termed CSF-1) and granulocyte-macrophage (GM)-CSF
macrophages in the liver adopt the features of resident Kupffer cells. have been studied extensively, but new details are still emerging. Both
The stromal macrophages associate with developing blood cells within transcription factor c-Myb and receptor FLT3, M-CSF–dependent mye-
16
islands of clustered cells, a feature of hematopoiesis throughout life. loid lineages (monocyte and dendritic) occur. These cell types may have
20
During fetal life, monocytes and macrophages are distributed through different responses to tissue damage and infection. Monocytes share
the developing vasculature, providing amoeboid, phagocytic cells precursors with other hematopoietic cells and are closely related to
17
implicated in tissue remodeling, for example, sculpting of digits, and granulocytes. Monocytic precursors are the source of adult tissue mac-
18
growth of the central nervous system. Blood monocytes seed resident rophages, as well as of myeloid DCs and osteoclasts. Their relationship
tissue macrophage populations throughout the organism, and these to B lymphocytes and to plasmacytoid DCs is still unclear, as plasmacy-
cells proliferate more readily in the fetus than in later life; the adhesion toid DCs express a range of myeloid as well as lymphoid markers. There
molecules, chemotactic signals, and receptors involved during this con- is a considerable body of knowledge about the specific growth factors
stitutive phase of distribution are poorly defined, but it is independent of and their receptors, and growing knowledge of the nature and role of
the β -integrin CD11b/CD18, which plays a role in myelomonocytic cell transcription factors involved in monocyte/macrophage differentia-
2
19
21
recruitment induced by inflammation in the adult. The appearance of tion. Genetic and cellular abnormalities in growth and differentiation
macrophages during development has been correlated with fibrous scar pathways underlie myeloid leukemogenesis, though rarely giving rise to
formation after injury. In sum, macrophages play a major role during monocytic leukemia.
17
Figure 68–1. Differentiation of the macrophage/dendritic cell (DC) progenitor and origin of macrophage and DC subsets. CDP, common dendritic
cell precursor; CMP, common myeloid progenitor; GMP, granulocyte/macrophage progenitor; HSC, hematopoietic stem cell; HSPCs, hematopoietic
stem and progenitor cells; MDP, macrophage/DC progenitor; pDC, plasmacytoid dendritic cell. For further details see Ref. 20. (Used with permission of
S. Seif, GraphisMedica, 2014.)
Kaushansky_chapter 68_p1075-1088.indd 1076 9/17/15 3:41 PM