Page 1186 - Williams Hematology ( PDFDrive )
P. 1186
1160 Part IX: Lymphocytes and Plasma Cells Chapter 75: Functions of B Lymphocytes and Plasma Cells in Immunoglobulin Production 1161
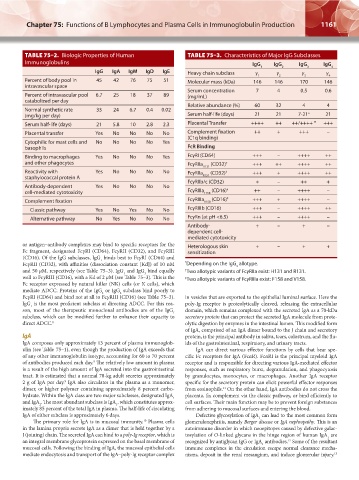
TABLE 75–2. Biologic Properties of Human TABLE 75–3. Characteristics of Major IgG Subclasses
Immunoglobulins IgG IgG IgG IgG
1 2 3 4
IgG IgA IgM IgD IgE Heavy chain subclass γ γ γ γ
4
1
2
3
Percent of body pool in 45 42 76 75 51 Molecular mass (kDa) 146 146 170 146
intravascular space
Serum concentration 7 4 0.5 0.6
Percent of intravascular pool 6.7 25 18 37 89 (mg/mL)
catabolized per day
Relative abundance (%) 60 32 4 4
Normal synthetic rate 33 24 6.7 0.4 0.02
(mg/kg per day) Serum half-life (days) 21 21 7-21 a 21
Serum half-life (days) 21 5.8 10 2.8 2.3 Placental Transfer ++++ ++ ++/++++ * +++
Placental transfer Yes No No No No Complement fixation ++ + +++ –
(C1q binding)
Cytophilic for mast cells and No No No No Yes
basophils FcR Binding
Binding to macrophages Yes No No No Yes FcγRI (CD64) +++ – ++++ ++
and other phagocytes FcγRIIa (CD32) † +++ ++ ++++ ++
H131
Reactivity with Yes No No No No FcγRIIa (CD32) † +++ + ++++ ++
staphylococcal protein A R131
FcγRIIb/c (CD32) + – ++ +
Antibody-dependent Yes No No No No
cell-mediated cytotoxicity FcγRIIIa F158 (CD16) ‡ ++ – ++++ –
Complement fixation FcγRIIIa V158 (CD16) ‡ +++ + ++++ –
Classic pathway Yes No Yes No No FcγRIIIb (CD16) +++ – ++++ ++
Alternative pathway No Yes No No No FcγRn (at pH <6.5) +++ – ++++ –
Antibody- + – + –
dependent cell-
mediated cytotoxicity
or antigen–antibody complexes may bind to specific receptors for the Heterologous skin + – + +
Fc fragment, designated FcγRI (CD64), FcγRII (CD32), and FcγRIII sensitization
(CD16). Of the IgG subclasses, IgG binds best to FcγRI (CD64) and
1
FcγRII (CD32), with affinities (dissociation constant [Kd]) of 10 nM * Depending on the IgG allotype.
3
and 50 μM, respectively (see Table 75–3). IgG and IgG bind equally † Two allotypic variants of FcγRIIa exist: H131 and R131.
3
1
well to FcγRIII (CD16), with a Kd of 2 μM (see Table 75–3). This is the ‡ Two allotypic variants of FcγRIIIa exist: F158 and V158.
Fc receptor expressed by natural killer (NK) cells (or K cells), which
mediate ADCC. Proteins of the IgG or IgG subclass bind poorly to
4
2
FcγRI (CD64) and bind not at all to FcγRIII (CD16) (see Table 75–3). in vesicles that are exported to the epithelial luminal surface. Here the
IgG is the most proficient subclass at directing ADCC. For this rea- poly-Ig receptor is proteolytically cleaved, releasing the extracellular
1
son, most of the therapeutic monoclonal antibodies are of the IgG domain, which remains complexed with the secreted IgA as a 70-kDa
1
subclass, which can be modified further to enhance their capacity to secretory protein that can protect the secreted IgA molecule from prote-
direct ADCC. 8 olytic digestion by enzymes in the intestinal lumen. This modified form
of IgA, comprised of an IgA dimer bound to the J chain and secretory
IgA protein, is the principal antibody in saliva, tears, colostrum, and the flu-
IgA composes only approximately 13 percent of plasma immunoglob- ids of the gastrointestinal, respiratory, and urinary tracts.
ulin (see Table 75–1), even though the production of IgA exceeds that IgA can direct various effector functions by cells that bear spe-
of any other immunoglobulin isotype, accounting for 60 to 70 percent cific Fc receptors for IgA (FcαR). FcαRI is the principal myeloid IgA
9
of antibodies produced each day. The relatively low amount in plasma receptor and is responsible for directing various IgA-mediated effector
is a result of the high amount of IgA secreted into the gastrointestinal responses, such as respiratory burst, degranulation, and phagocytosis
tract. It is estimated that a normal 70-kg adult secretes approximately by granulocytes, monocytes, or macrophages. Another IgA receptor
9
2 g of IgA per day. IgA also circulates in the plasma as a monomer, specific for the secretory protein can elicit powerful effector responses
dimer, or higher polymer containing approximately 8 percent carbo- from eosinophils. On the other hand, IgA antibodies do not cross the
11
hydrate. Within the IgA class are two major subclasses, designated IgA 1 placenta, fix complement via the classic pathway, or bind efficiently to
and IgA . The most abundant subclass is IgA , which constitutes approx- cell surfaces. Their main function may be to prevent foreign substances
2
1
imately 85 percent of the total IgA in plasma. The half-life of circulating from adhering to mucosal surfaces and entering the blood.
IgA of either subclass is approximately 6 days. Defective glycosylation of IgA can lead to the most common form
1
The primary role for IgA is in mucosal immunity. Plasma cells glomerulonephritis, namely Berger disease or IgA nephropathy. This is an
10
in the lamina propria secrete IgA as a dimer that is held together by a autoimmune disorder in which neoepitopes caused by defective galac-
J (joining) chain. The secreted IgA can bind to a poly-Ig receptor, which is tosylation of O-linked glycans in the hinge region of human IgA are
1
an integral membrane glycoprotein expressed on the basal membrane of recognized by antiglycan IgG or IgA antibodies. Some of the resultant
12
1
mucosal cells. Following the binding of IgA, the mucosal epithelial cells immune complexes in the circulation escape normal clearance mecha-
mediate endocytosis and transport of the IgA–poly-Ig receptor complex nisms, deposit in the renal mesangium, and induce glomerular injury.
13
Kaushansky_chapter 75_p1159-1174.indd 1161 9/21/15 12:10 PM