Page 1536 - Williams Hematology ( PDFDrive )
P. 1536
1510 Part XI: Malignant Lymphoid Diseases Chapter 91: Acute Lymphoblastic Leukemia 1511
are better than blood cells for genetic studies. Fibrosis or tightly packed Cytochemical stains (Sudan black stain and the stains for myelop-
marrow can lead to difficulties with marrow aspiration that necessitate eroxidase and the nonspecific esterases) distinguish ALL from acute
biopsy. In patients with marrow necrosis, multiple marrow aspirations myeloid leukemia but are now less commonly used for diagnosis than
are sometimes needed to obtain diagnostic tissue. immunophenotyping.
Immunologic Classification
Morphologic and Cytochemical Analysis Immunophenotyping is an essential part of the diagnostic evaluation.
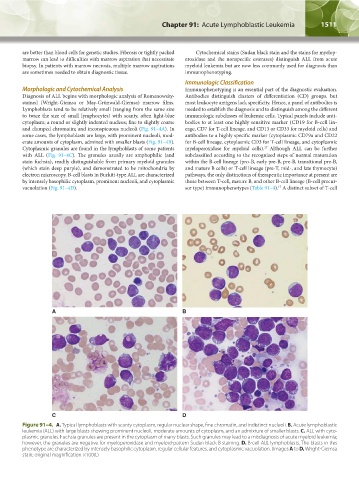
Diagnosis of ALL begins with morphologic analysis of Romanowsky- Antibodies distinguish clusters of differentiation (CD) groups, but
stained (Wright-Giemsa or May-Grünwald-Giemsa) marrow films. most leukocyte antigens lack specificity. Hence, a panel of antibodies is
Lymphoblasts tend to be relatively small (ranging from the same size needed to establish the diagnosis and to distinguish among the different
to twice the size of small lymphocytes) with scanty, often light-blue immunologic subclasses of leukemic cells. Typical panels include anti-
cytoplasm; a round or slightly indented nucleus; fine to slightly coarse bodies to at least one highly sensitive marker (CD19 for B-cell lin-
and clumped chromatin; and inconspicuous nucleoli (Fig. 91–4A). In eage, CD7 for T-cell lineage, and CD13 or CD33 for myeloid cells) and
some cases, the lymphoblasts are large, with prominent nucleoli, mod- antibodies to a highly specific marker (cytoplasmic CD79a and CD22
erate amounts of cytoplasm, admixed with smaller blasts (Fig. 91–4B). for B-cell lineage, cytoplasmic CD3 for T-cell lineage, and cytoplasmic
17
Cytoplasmic granules are found in the lymphoblasts of some patients myeloperoxidase for myeloid cells). Although ALL can be further
with ALL (Fig. 91–4C). The granules usually are amphophilic (and subclassified according to the recognized steps of normal maturation
stain fuchsia), readily distinguishable from primary myeloid granules within the B-cell lineage (pro-B, early pre-B, pre-B, transitional pre-B,
(which stain deep purple), and demonstrated to be mitochondria by and mature B cells) or T-cell lineage (pre-T, mid-, and late thymocyte)
electron microscopy. B-cell blasts in Burkitt-type ALL are characterized pathways, the only distinctions of therapeutic importance at present are
by intensely basophilic cytoplasm, prominent nucleoli, and cytoplasmic those between T-cell, mature B, and other B-cell lineage (B-cell precur-
17
vacuolation (Fig. 91–4D). sor type) immunophenotypes (Table 91–4). A distinct subset of T-cell
A B
C D
Figure 91–4. A. Typical lymphoblasts with scanty cytoplasm, regular nuclear shape, fine chromatin, and indistinct nucleoli. B. Acute lymphoblastic
leukemia (ALL) with large blasts showing prominent nucleoli, moderate amounts of cytoplasm, and an admixture of smaller blasts. C. ALL with cyto-
plasmic granules. Fuchsia granules are present in the cytoplasm of many blasts. Such granules may lead to a misdiagnosis of acute myeloid leukemia;
however, the granules are negative for myeloperoxidase and myeloid-pattern Sudan black B staining. D. B-cell ALL lymphoblasts. The blasts in this
phenotype are characterized by intensely basophilic cytoplasm, regular cellular features, and cytoplasmic vacuolation. (Images A to D, Wright-Giemsa
stain; original magnification ×1000.)
Kaushansky_chapter 91_p1505-1526.indd 1511 9/21/15 12:19 PM