Page 1680 - Williams Hematology ( PDFDrive )
P. 1680
1654 Part XI: Malignant Lymphoid Diseases Chapter 100: Mantle Cell Lymphoma 1655
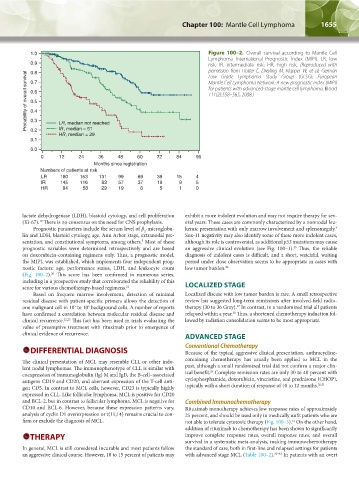
1.0 Figure 100–2. Overall survival according to Mantle Cell
Lymphoma International Prognostic Index (MIPI). LR, low
0.9 risk; IR, intermediate risk; HR, high risk. (Reproduced with
permission from Hoster E, Dreyling M, Klapper W, et al: German
0.8
Probability of overall survival 0.7 Mantle Cell Lymphoma Network: A new prognostic index (MIPI)
Low Grade Lymphoma Study Group (GLSG); European
for patients with advanced-stage mantle cell lymphoma. Blood
0.6
111(2):558–565, 2008.)
0.5
0.4
0.3
LR, median not reached
IR, median = 51
0.2
HR, median = 29
0.1
0.0
0 12 24 36 48 60 72 84 96
Months since registration
Numbers of patients at risk
LR 180 153 131 99 69 39 15 4
IR 145 116 83 57 37 19 9 5
HR 84 58 29 19 8 5 1 0
lactate dehydrogenase (LDH), blastoid cytology, and cell proliferation exhibit a more indolent evolution and may not require therapy for sev-
(Ki-67). There is no consensus on the need for CNS prophylaxis. eral years. These cases are commonly characterized by a nonnodal leu-
19
Prognostic parameters include the serum level of β -microglobu- kemic presentation with only marrow involvement and splenomegaly.
4
2
lin and LDH, blastoid cytology, age, Ann Arbor stage, extranodal pre- Sox-11 negativity may also identify some of these more indolent cases,
sentation, and constitutional symptoms, among others. Most of these although its role is controversial, as additional p53 mutations may cause
5
prognostic variables were determined retrospectively and are based an aggressive clinical evolution (see Fig. 100–1). Thus, the reliable
25
on doxorubicin-containing regimens only. Thus, a prognostic model, diagnosis of indolent cases is difficult, and a short, watchful, waiting
the MIPI, was established, which implements four independent prog- period under close observation seems to be appropriate in cases with
nostic factors: age, performance status, LDH, and leukocyte count low tumor burden. 26
(Fig. 100–2). This score has been confirmed in numerous series,
20
including in a prospective study that corroborated the reliability of this
score for various chemotherapy-based regimens. 21 LOCALIZED STAGE
Based on frequent marrow involvement, detection of minimal Localized disease with low tumor burden is rare. A small retrospective
residual disease with patient-specific primers allows the detection of review has suggested long-term remissions after involved-field radio-
one malignant cell in 10 to 10 background cells. A number of reports therapy (30 to 36 Gray). In contrast, in a randomized trial all patients
27
6
5
28
have confirmed a correlation between molecular residual disease and relapsed within a year. Thus, a shortened chemotherapy induction fol-
clinical recurrence. 22,23 This fact has been used in trials evaluating the lowed by radiation consolidation seems to be most appropriate.
value of preemptive treatment with rituximab prior to emergence of
clinical evidence of recurrence. ADVANCED STAGE
DIFFERENTIAL DIAGNOSIS Conventional Chemotherapy
Because of the typical aggressive clinical presentation, anthracycline-
The clinical presentation of MCL may resemble CLL or other indo- containing chemotherapy has usually been applied to MCL in the
lent nodal lymphomas. The immunophenotype of CLL is similar with past, although a small randomized trial did not confirm a major clin-
29
coexpression of immunoglobulin (Ig) M and IgD, the B-cell–associated ical benefit. Complete remission rates are only 30 to 40 percent with
antigens CD19 and CD20, and aberrant expression of the T-cell anti- cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP),
gen CD5. In contrast to MCL cells, however, CD23 is typically highly typically with a short duration of response of 10 to 12 months. 30,31
expressed in CLL. Like follicular lymphoma, MCL is positive for CD20
and BCL-2, but in contrast to follicular lymphoma, MCL is negative for Combined Immunochemotherapy
CD10 and BCL-6. However, because these expression patterns vary, Rituximab monotherapy achieves low response rates of approximately
analysis of cyclin D1 overexpression or t(11;14) remains crucial to con- 25 percent, and should be used only in medically unfit patients who are
firm or exclude the diagnosis of MCL. not able to tolerate cytotoxic therapy (Fig. 100–3). On the other hand,
32
addition of rituximab to chemotherapy has been shown to significantly
THERAPY improve complete response rates, overall response rates, and overall
survival in a systematic meta-analysis, making immunochemotherapy
In general, MCL is still considered incurable and most patients follow the standard of care, both in first-line and relapsed settings for patients
an aggressive clinical course. However, 10 to 15 percent of patients may with advanced stage MCL (Table 100–2). 33–43 In patients with an overt
Kaushansky_chapter 100_p1653-1662.indd 1655 9/18/15 5:06 PM