Page 1683 - Williams Hematology ( PDFDrive )
P. 1683
1658 Part XI: Malignant Lymphoid Diseases Chapter 100: Mantle Cell Lymphoma 1659
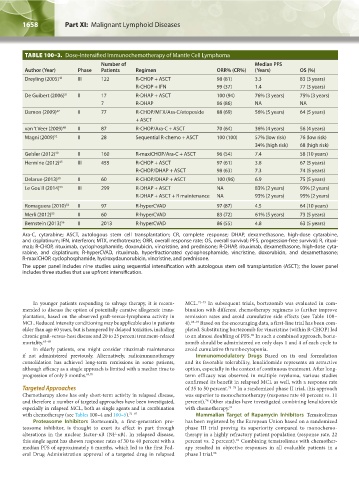
TABLE 100–3. Dose-Intensified Immunochemotherapy of Mantle Cell Lymphoma
Number of Median PFS
Author (Year) Phase Patients Regimen ORR% (CR%) (Years) OS (%)
Dreyling (2005) 46 III 122 R-CHOP + ASCT 98 (81) 3.3 83 (3 years)
R-CHOP + IFN 99 (37) 1.4 77 (3 years)
De Guibert (2006) 51 II 17 R-DHAP + ASCT 100 (94) 76% (3 years) 75% (3 years)
7 R-DHAP 86 (86) NA NA
Damon (2009) 47 II 77 R-CHOP/MTX/Ara-C/etoposide 88 (69) 56% (5 years) 64 (5 years)
+ ASCT
van ‘t Veer (2009) 48 II 87 R-CHOP/Ara-C + ASCT 70 (64) 36% (4 years) 56 (4 years)
Magni (2009) 52 II 28 Sequential R-chemo + ASCT 100 (100) 57% (low risk) 76 (low risk)
34% (high risk) 68 (high risk)
Geisler (2012) 49 II 160 R-maxiCHOP/Ara-C + ASCT 96 (54) 7.4 58 (10 years)
Hermine (2012) 45 III 455 R-CHOP + ASCT 97 (61) 3.8 67 (5 years)
R-CHOP/DHAP + ASCT 98 (63) 7.3 74 (5 years)
Delarue (2013) 50 II 60 R-CHOP/DHAP + ASCT 100 (96) 6.9 75 (5 years)
Le Gouill (2014) 53 III 299 R-DHAP + ASCT NA 83% (2 years) 93% (2 years)
R-DHAP + ASCT + R maintenance NA 93% (2 years) 95% (2 years)
Romaguera (2010) 54 II 97 R-hyperCVAD 97 (87) 4.5 64 (10 years)
Merli (2012) 55 II 60 R-hyperCVAD 83 (72) 61% (5 years) 73 (5 years)
Bernstein (2013) 56 II 2013 R-hyperCVAD 86 (55) 4.8 63 (5 years)
Ara-C, cytarabine; ASCT, autologous stem cell transplantation; CR, complete response; DHAP, dexamethasone, high-dose cytarabine,
and cisplatinum; IFN, interferon; MTX, methotrexate; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; R, rituxi-
mab; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-DHAP, rituximab, dexamethasone, high-dose cyta-
rabine, and cisplatinum; R-hyperCVAD, rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone;
R-maxiCHOP, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone.
The upper panel includes nine studies using sequential intensification with autologous stem cell transplantation (ASCT); the lower panel
includes three studies that use upfront intensification.
In younger patients responding to salvage therapy, it is recom- MCL. 71–73 In subsequent trials, bortezomib was evaluated in com-
mended to discuss the option of potentially curative allogeneic trans- bination with different chemotherapy regimens to further improve
plantation, based on the observed graft-versus-lymphoma activity in remission rates and avoid cumulative side effects (see Table 100–
MCL. Reduced intensity conditioning may be applicable also in patients 4). 88–93 Based on the encouraging data, a first-line trial has been com-
older than age 60 years, but is hampered by delayed toxicities, including pleted. Substituting bortezomib for vincristine (within R-CHOP) led
chronic graft-versus-host disease and 20 to 25 percent treatment-related to an almost doubling of PFS. In such a combined approach, borte-
98
mortality. 65–68 zomib should be administered on only days 1 and 4 of each cycle to
In elderly patients, one might consider rituximab maintenance avoid cumulative thrombocytopenia.
if not administered previously. Alternatively, radioimmunotherapy Immunomodulatory Drugs Based on its oral formulation
consolidation has achieved long-term remissions in some patients, and its favorable tolerability, lenalidomide represents an attractive
although efficacy as a single approach is limited with a median time to option, especially in the context of continuous treatment. After long-
progression of only 5 months. 69,70 term efficacy was observed in multiple myeloma, various studies
confirmed its benefit in relapsed MCL as well, with a response rate
Targeted Approaches of 35 to 50 percent. 75–78 In a randomized phase II trial, this approach
Chemotherapy alone has only short-term activity in relapsed disease, was superior to monochemotherapy (response rate 40 percent vs. 11
and therefore a number of targeted approaches have been investigated, percent). Other studies have investigated combining lenalidomide
78
especially in relapsed MCL, both as single agents and in combination with chemotherapy. 94
with chemotherapy (see Tables 100–4 and 100–5). 71–97 Mammalian Target of Rapamycin Inhibitors Temsirolimus
Proteasome Inhibitors Bortezomib, a first-generation pro- has been registered by the European Union based on a randomized
teosome inhibitor, is thought to exert its effect in part through phase III trial proving its superiority compared to monochemo-
alterations in the nuclear factor-κB (NF-κB). In relapsed disease, therapy in a highly refractory patient population (response rate, 22
this single agent has shown response rates of 30 to 40 percent with a percent vs. 2 percent). Combining temsirolimus with chemother-
81
median PFS of approximately 6 months, which led to the first Fed- apy resulted in objective responses in all evaluable patients in a
eral Drug Administration approval of a targeted drug in relapsed phase I trial. 96
Kaushansky_chapter 100_p1653-1662.indd 1658 9/18/15 5:06 PM