Page 1778 - Williams Hematology ( PDFDrive )
P. 1778
1752 Part XI: Malignant Lymphoid Diseases Chapter 107: Myeloma 1753
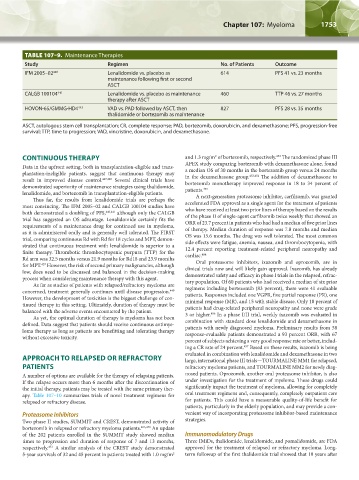
TABLE 107–9. Maintenance Therapies
Study Regimen No. of Patients Outcome
IFM 2005–02 440 Lenalidomide vs. placebo as 614 PFS 41 vs. 23 months
maintenance following first or second
ASCT
CALGB 100104 441 Lenalidomide vs. placebo as maintenance 460 TTP 46 vs. 27 months
therapy after ASCT
HOVON-65/GMMG-HD4 443 VAD vs. PAD followed by ASCT, then 827 PFS 28 vs. 35 months
thalidomide or bortezomib as maintenance
ASCT, autologous stem cell transplantation; CR, complete response; PAD, bortezomib, doxorubicin, and dexamethasone; PFS, progression-free
survival; TTP, time to progression; VAD, vincristine, doxorubicin, and dexamethasone.
CONTINUOUS THERAPY and 1.3 mg/m of bortezomib, respectively. The randomized phase III
2
452
APEX study comparing bortezomib with dexamethasone alone, found
Data in the upfront setting, both in transplantation-eligible and trans- a median OS of 30 months in the bortezomib group versus 24 months
plantation-ineligible patients, suggest that continuous therapy may in the dexamethasone group. 453,454 The addition of dexamethasone to
result in improved disease control. 447,448 Several clinical trials have bortezomib monotherapy improved response in 18 to 34 percent of
demonstrated superiority of maintenance strategies using thalidomide, patients. 455
lenalidomide, and bortezomib in transplantation-eligible patients. A next-generation proteasome inhibitor, carfilzomib, was granted
Thus far, the results from lenalidomide trials are perhaps the accelerated FDA approval as a single agent for the treatment of patients
most convincing. The IFM 2005–02 and CALGB 100104 studies have who have received at least two prior lines of therapy based on the results
both demonstrated a doubling of PFS, 440,441 although only the CALGB of the phase II of single-agent carfilzomib twice weekly that showed an
trial has suggested an OS advantage. Lenalidomide certainly fits the ORR of 23.7 percent in patients who had had a median of five prior lines
requirements of a maintenance drug for continued use in myeloma, of therapy. Median duration of response was 7.8 months and median
as it is administered orally and is generally well tolerated. The FIRST OS was 15.6 months. The drug was well tolerated. The most common
trial, comparing continuous Rd with Rd for 18 cycles and MPT, demon- side effects were fatigue, anemia, nausea, and thrombocytopenia, with
strated that continuous treatment with lenalidomide is superior to a 12.4 percent reporting treatment-related peripheral neuropathy and
finite therapy. Thrombotic thrombocytopenic purpura (TTP) for the cardiac. 404
Rd arm was 32.5 months versus 21.9 months for Rd18 and 23.9 months Oral proteasome inhibitors, ixazomib and oprozomib, are in
437
for MPT. However, the risk of second primary malignancies, although clinical trials now and will likely gain approval. Ixazomib, has already
low, does need to be discussed and balanced in the decision-making demonstrated safety and efficacy in phase I trials in the relapsed, refrac-
process when considering maintenance therapy with this agent. tory population. Of 60 patients who had received a median of six prior
As far as studies of patients with relapsed/refractory myeloma are regimens including bortezomib (83 percent), there were 41 evaluable
449
concerned, treatment generally continues until disease progression. patients. Responses included one VGPR, five partial response (PR), one
However, the development of toxicities is the biggest challenge of con- minimal response (MR), and 15 with stable disease. Only 10 percent of
tinued therapy in this setting. Ultimately, duration of therapy must be patients had drug-related peripheral neuropathy and none were grade
balanced with the adverse events encountered by the patient. 3 or higher. In a phase I/II trial, weekly ixazomib was evaluated in
456
As yet, the optimal duration of therapy is myeloma has not been combination with standard dose lenalidomide and dexamethasone in
defined. Data suggest that patients should receive continuous antimye- patients with newly diagnosed myeloma. Preliminary results from 58
loma therapy as long as patients are benefitting and tolerating therapy response-evaluable patients demonstrated a 93 percent ORR, with 67
without excessive toxicity.
percent of subjects achieving a very good response rate or better, includ-
ing a CR rate of 24 percent. Based on these results, ixazomib is being
457
evaluated in combination with lenalidomide and dexamethasone in two
APPROACH TO RELAPSED OR REFRACTORY large, international phase III trials—TOURMALINE MM1 for relapsed,
PATIENTS refractory myeloma patients, and TOURMALINE MM2 for newly diag-
A number of options are available for the therapy of relapsing patients. nosed patients. Oprozomib, another oral proteasome inhibitor, is also
If the relapse occurs more than 6 months after the discontinuation of under investigation for the treatment of myeloma. These drugs could
the initial therapy, patients may be treated with the same primary ther- significantly impact the treatment of myeloma, allowing for completely
apy. Table 107–10 summarizes trials of novel treatment regimens for oral treatment regimens and, consequently, completely outpatient care
relapsed or refractory disease. for patients. This could have a measurable quality-of-life benefit for
patients, particularly in the elderly population, and may provide a con-
Proteasome Inhibitors venient way of incorporating proteasome inhibitor-based maintenance
Two phase II studies, SUMMIT and CREST, demonstrated activity of strategies.
bortezomib in relapsed or refractory myeloma patients. 403,450 An update
of the 202 patients enrolled in the SUMMIT study showed median Immunomodulatory Drugs
times to progression and duration of response of 7 and 13 months, Three IMiDs, thalidomide, lenalidomide, and pomalidomide, are FDA
451
respectively. A similar analysis of the CREST study demonstrated approved for the treatment of relapsed or refractory myeloma. Long-
2
5-year survivals of 32 and 45 percent in patients treated with 1.0 mg/m term followup of the first thalidomide trial showed that 10 years after
Kaushansky_chapter 107_p1733-1772.indd 1753 9/21/15 12:35 PM