Page 1775 - Williams Hematology ( PDFDrive )
P. 1775
1750 Part XI: Malignant Lymphoid Diseases Chapter 107: Myeloma 1751
High-dose chemoradiotherapy followed by transplantation of
either autologous marrow or PBPCs has achieved high (40 percent) com-
plete response (CR) rates, but the median duration of these responses
has been only 2 to 3 years. The Intergroupe Francais du Myelome (IFM
90), a national French study, first demonstrated the efficacy of auto-
HSCT over conventional chemotherapy in 200 myeloma patients.
399
Several randomized trials and case-controlled studies have been per-
formed and the results have been variable. For example, the Medical
Research Council (MRC) randomized study confirmed a 12-month
408
survival benefit for the transplanted arm. In contrast, the U.S. Inter-
group randomized trial was unable to confirm the benefits of transplan-
409
tation. Despite the use of aggressive approaches like transplantation,
few, if any, patients are cured. To improve upon the results of high-dose
chemotherapy, the French group has compared single versus double
A B autografts and their data suggest that two sequential transplants may
benefit a subset of patients with myeloma who did not achieve a com-
410
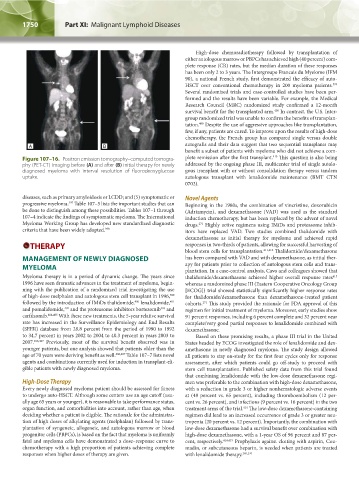
Figure 107–16. Positron emission tomography–computed tomogra- plete remission after the first transplant. This question is also being
phy (PET-CT) imaging before (A) and after (B) initial therapy for newly addressed by the ongoing phase III, multicenter trial of single autolo-
diagnosed myeloma with interval resolution of fluorodeoxyglucose gous transplant with or without consolidation therapy versus tandem
uptake. autologous transplant with lenalidomide maintenance (BMT CTN
0702).
diseases, such as primary amyloidosis or LCDD; and (5) symptomatic or Novel Agents
progressive myeloma. Table 107–5 lists the important studies that can Beginning in the 1980s, the combination of vincristine, doxorubicin
397
be done to distinguish among these possibilities. Tables 107–1 through (Adriamycin), and dexamethasone (VAD) was used as the standard
107–4 indicate the findings of symptomatic myeloma. The International induction chemotherapy, but has been replaced by the advent of novel
Myeloma Working Group has developed new standardized diagnostic drugs. Highly active regimens using IMiDs and proteasome inhib-
271
criteria that have been widely adopted. 398 itors have replaced VAD. Two studies combined thalidomide with
dexamethasone as initial therapy for myeloma and achieved rapid
THERAPY responses in two-thirds of patients, allowing for successful harvesting of
blood stem cells for transplantation. 411,412 Thalidomide/dexamethasone
MANAGEMENT OF NEWLY DIAGNOSED has been compared with VAD and with dexamethasone, as initial ther-
MYELOMA apy for patients prior to collection of autologous stem cells and trans-
plantation. In a case-control analysis, Cavo and colleagues showed that
Myeloma therapy is in a period of dynamic change. The years since thalidomide/dexamethasone achieved higher overall response rates
413
1996 have seen dramatic advances in the treatment of myeloma, begin- whereas a randomized phase III (Eastern Cooperative Oncology Group
ning with the publication of a randomized trial investigating the use [ECOG]) trial showed statistically significantly higher response rates
of high-dose melphalan and autologous stem cell transplant in 1996, for thalidomide/dexamethasone than dexamethasone-treated patient
399
followed by the introduction of IMiDs thalidomide, lenalidomide, cohorts. This study provided the rationale for FDA approval of this
401
400
271
and pomalidomide, and the proteasome inhibitors bortezomib and regimen for initial treatment of myeloma. Moreover, early studies show
403
402
carfilzomib. 404,405 With these new treatments, the 5-year relative survival 91 percent responses, including 6 percent complete and 32 percent near
rate has increased in the Surveillance Epidemiology and End Results complete/very good partial responses to lenalidomide combined with
(SEER) database from 28.8 percent from the period of 1990 to 1992 dexamethasone.
to 34.7 percent in years 2002 to 2004 to 40.3 percent in years 2003 to Based on these promising results, a phase III trial in the United
2007. 406,407 Previously, most of the survival benefit observed was in States headed by ECOG investigated the role of lenalidomide and dex-
younger patients, but one analysis showed that patients older than the amethasone in newly diagnosed myeloma. The study design allowed
age of 70 years were deriving benefit as well. 406,407 Table 107–7 lists novel all patients to stay on-study for the first four cycles only for response
agents and combinations currently used for induction in transplant-eli- assessment, after which patients could go off-study to proceed with
gible patients with newly diagnosed myeloma. stem cell transplantation. Published safety data from this trial found
that combining lenalidomide with the low-dose dexamethasone regi-
High-Dose Therapy men was preferable to the combination with high-dose dexamethasone,
Every newly diagnosed myeloma patient should be assessed for fitness with a reduction in grade 3 or higher nonhematologic adverse events
to undergo auto-HSCT. Although some centers use an age cutoff (usu- at (48 percent vs. 65 percent), including thromboembolism (12 per-
ally age 65 years or younger), it is reasonable to take performance status, cent vs. 26 percent), and infections (9 percent vs. 16 percent) in the two
organ function, and comorbidities into account, rather than age, when treatment arms of the trial. The low-dose dexamethasone-containing
414
deciding whether a patient is eligible. The rationale for the administra- regimen did lead to an increased occurrence of grade 3 or greater neu-
tion of high doses of alkylating agents (melphalan) followed by trans- tropenia (20 percent vs. 12 percent). Importantly, the combination with
plantation of syngeneic, allogeneic, and autologous marrow or blood low-dose dexamethasone had a survival benefit over combination with
progenitor cells (PBPCs), is based on the fact that myeloma is uniformly high-dose dexamethasone, with a 1-year OS of 96 percent and 87 per-
fatal and myeloma cells have demonstrated a dose–response curve to cent, respectively. 414,415 Prophylaxis against clotting with aspirin, Cou-
chemotherapy with a high proportion of patients achieving complete madin, or subcutaneous heparin, is needed when patients are treated
responses when higher doses of therapy are given. with lenalidomide therapy. 269,270
Kaushansky_chapter 107_p1733-1772.indd 1750 9/21/15 12:35 PM