Page 1779 - Williams Hematology ( PDFDrive )
P. 1779
1754 Part XI: Malignant Lymphoid Diseases Chapter 107: Myeloma 1755
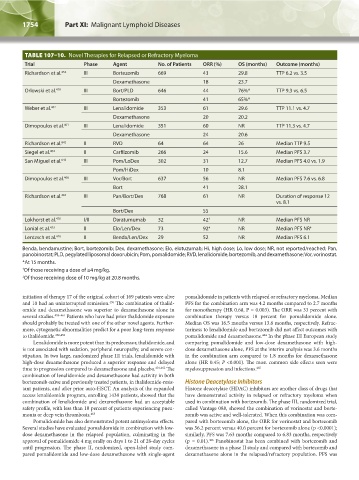
TABLE 107–10. Novel Therapies for Relapsed or Refractory Myeloma
Trial Phase Agent No. of Patients ORR (%) OS (months) Outcome (months)
Richardson et al. 454 III Bortezomib 669 43 29.8 TTP 6.2 vs. 3.5
Dexamethasone 18 23.7
Orlowski et al. 479 III Bort/PLD 646 44 76%* TTP 9.3 vs. 6.5
Bortezomib 41 65%*
Weber et al. 462 III Lenalidomide 353 61 29.6 TTP 11.1 vs. 4.7
Dexamethasone 20 20.2
Dimopoulos et al. 401 III Lenalidomide 351 60 NR TTP 11.3 vs. 4.7
Dexamethasone 24 20.6
Richardson et al. 642 II RVD 64 64 26 Median TTP 9.5
Siegel et al. 404 II Carfilzomib 266 24 15.6 Median PFS 3.7
San Miguel et al. 643 III Pom/LoDex 302 31 12.7 Median PFS 4.0 vs. 1.9
Pom/HiDex 10 8.1
Dimopoulos et al. 466 III Vor/Bort 637 56 NR Median PFS 7.6 vs. 6.8
Bort 41 28.1
Richardson et al. 467 III Pan/Bort/Dex 768 61 NR Duration of response 12
vs. 8.1
Bort/Dex 55
Lokhorst et al. 470 I/II Daratumumab 32 42 † NR Median PFS NR
Lonial et al. 473 II Elo/Len/Dex 73 92 ‡ NR Median PFS NR ‡
Lentzsch et al. 476 II Benda/Len/Dex 29 52 NR Median PFS 6.1
Benda, bendamustine; Bort, bortezomib; Dex, dexamethasone; Elo, elotuzumab; Hi, high dose; Lo, low dose; NR, not reported/reached; Pan,
panobinostat; PLD, pegylated liposomal doxorubicin; Pom, pomalidomide; RVD, lenalidomide, bortezomib, and dexamethasone; Vor, vorinostat.
*At 15 months.
† Of those receiving a dose of ≥4 mg/kg.
‡ Of those receiving dose of 10 mg/kg at 20.8 months.
initiation of therapy 17 of the original cohort of 169 patients were alive pomalidomide in patients with relapsed or refractory myeloma. Median
and 10 had an uninterrupted remission. The combination of thalid- PFS for the combination arm was 4.2 months compared to 2.7 months
458
omide and dexamethasone was superior to dexamethasone alone in for monotherapy (HR 0.68, P = 0.003). The ORR was 33 percent with
several studies. 459–461 Patients who have had prior thalidomide exposure combination therapy versus 18 percent for pomalidomide alone.
should probably be treated with one of the other novel agents. Further- Median OS was 16.5 months versus 13.6 months, respectively. Refrac-
more, cytogenetic abnormalities predict for a poor long-term response toriness to lenalidomide and bortezomib did not affect outcomes with
to thalidomide. 268,458 pomalidomide and dexamethasone. In the phase III European study
464
Lenalidomide is more potent than its predecessor, thalidomide, and comparing pomalidomide and low-dose dexamethasone with high-
is not associated with sedation, peripheral neuropathy, and severe con- dose dexamethasone alone, PFS at the interim analysis was 3.6 months
stipation. In two large, randomized phase III trials, lenalidomide with in the combination arm compared to 1.8 months for dexamethasone
high-dose dexamethasone produced a superior response and delayed alone (HR 0.45; P <0.001). The most common side effects seen were
time to progression compared to dexamethasone and placebo. 401,462 The myelosuppression and infections. 465
combination of lenalidomide and dexamethasone had activity in both
bortezomib-naïve and previously treated patients, in thalidomide-resis- Histone Deacetylase Inhibitors
tant patients, and after prior auto-HSCT. An analysis of the expanded Histone deacetylase (HDAC) inhibitors are another class of drugs that
access lenalidomide program, enrolling 1438 patients, showed that the have demonstrated activity in relapsed or refractory myeloma when
combination of lenalidomide and dexamethasone had an acceptable used in combination with bortezomib. The phase III, randomized trial,
safety profile, with less than 10 percent of patients experiencing pneu- called Vantage 088, showed the combination of vorinostat and borte-
monia or deep vein thrombosis. 463 zomib was active and well-tolerated. When this combination was com-
Pomalidomide has also demonstrated potent antimyeloma effects. pared with bortezomib alone, the ORR for vorinostat and bortezomib
Several studies have evaluated pomalidomide in combination with low- was 56.2 percent versus 40.6 percent for bortezomib alone (p <0.0001);
dose dexamethasone in the relapsed population, culminating in the similarly, PFS was 7.63 months compared to 6.83 months, respectively
approval of pomalidomide 4 mg orally on days 1 to 21 of 28-day cycles (p = 0.01). Panobinostat has been combined with bortezomib and
466
until progression. The phase II, randomized, open-label study com- dexamethasone in a phase II study and compared with bortezomib and
pared pomalidomide and low-dose dexamethasone with single-agent dexamethasone alone in the relapsed/refractory population. PFS was
Kaushansky_chapter 107_p1733-1772.indd 1754 9/21/15 12:35 PM