Page 1885 - Williams Hematology ( PDFDrive )
P. 1885
1860 Part XII: Hemostasis and Thrombosis Chapter 112: Platelet Morphology, Biochemistry, and Function 1861
Inside-out Ligand
signaling binding
A B C
Traction
Talin F3
A domain
B C
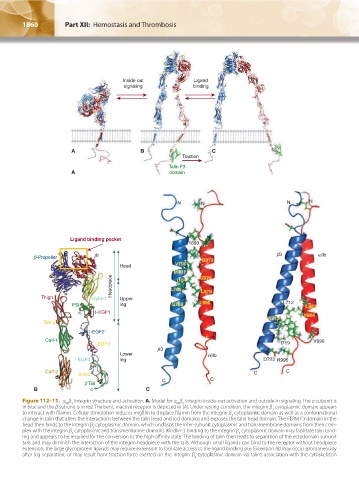
Figure 112–11. α β Integrin structure and activation. A. Model for α β integrin inside-out activation and outside-in signaling. The α subunit is
IIb 3
IIb 3
in blue and the β subunit is in red. The bent, inactive receptor is depicted in (A). Under resting condition, the integrin β cytoplasmic domain appears
3
to interact with filamin. Cellular stimulation induces migfilin to displace filamin from the integrin β cytoplasmic domain as well as a conformational
3
change in talin that alters the interactions between the talin head and rod domains and exposes the talin head domain. The FERM F3 domain in the
head then binds to the integrin β cytoplasmic domain, which unclasps the inter-subunit cytoplasmic and transmembrane domains from their com-
3
plex with the integrin β cytoplasmic and transmembrane domains. Kindlin-3 binding to the integrin β cytoplasmic domain may facilitate talin bind-
3
3
ing and appears to be required for the conversion to the high-affinity state. The binding of talin then leads to separation of the ectodomain subunit
tails and may diminish the interaction of the integrin headpiece with the tails. Although small ligands can bind to the receptor without headpiece
extension, the large glycoprotein ligands may require extension to facilitate access to the ligand binding site. Extension (B) may occur spontaneously
after leg separation, or may result from traction force exerted on the integrin β cytoplasmic domain via talin’s association with the cytoskeleton
3
Kaushansky_chapter 112_p1829-1914.indd 1860 17/09/15 3:29 pm