Page 2261 - Williams Hematology ( PDFDrive )
P. 2261
2236 Part XII: Hemostasis and Thrombosis Chapter 131: The Antiphospholipid Syndrome 2237
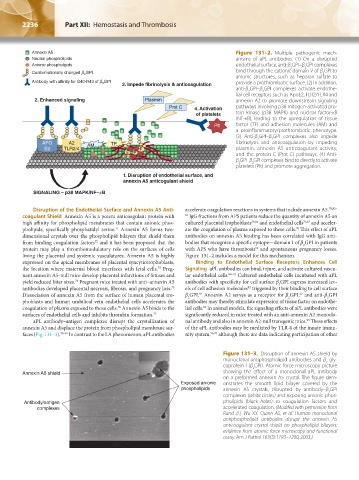
Annexin A5 Figure 131–2. Multiple pathogenic mech-
Neutral phospholipids anisms of aPL antibodies. (1) On a disrupted
Anionic phospholipids endothelial surface, anti-β GPI–β GPI complexes
2
2
Conformationally changed b GPI bind through the cationic domain V of β GPI to
2
2
anionic structures, such as heparan sulfate to
Antibody with affinity for G40-R43 of b GPI 3. Impede fibrinolysis & anticoagulation provide a prothrombotic surface. (2) In addition,
2
anti-β GPI–β GPI complexes activate endothe-
2
2
lial cell receptors such as ApoE2, TLR2/TLR4 and
2. Enhanced signaling Plasmin annexin A2 to promote downstream signaling
Prot C 4. Activation pathways involving p38 mitogen- activated pro-
of platelets tein kinase (p38 MAPK) and nuclear factor-κB
(NF-κB), leading to the upregulation of tissue
Plt factor (TF) and adhesion molecules (AM) and
a proinflammatory/prothrombotic phenotype.
(3) Anti-β GPI–β GPI complexes also impede
2
2
APO A2 AM TF fibrinolysis and anticoagulation by impeding
E2 TLR2/4 F plasmin, annexin A5 anticoagulant activity,
F F and the protein C (Prot C) pathways. (4) Anti-
F
β GPI–β GPI complexes bind to directly to activate
2
2
platelets (Plt) and promote aggregation.
1. Disruption of endothelial surface, and
annexin A5 anticogulant shield
SIGNALING – p38 MAPK/NF–kB
Disruption of the Endothelial Surface and Annexin A5 Anti- accelerate coagulation reactions in systems that include annexin A5. 78,82–
coagulant Shield Annexin A5 is a potent anticoagulant protein with 85 IgG fractions from APS patients reduce the quantity of annexin A5 on
high affinity for phospholipid membranes that contain anionic phos- cultured placental trophoblasts 76,86 and endothelial cells 76,87 and acceler-
pholipids, specifically phosphatidyl serine. Annexin A5 forms two- ate the coagulation of plasma exposed to these cells. This effect of aPL
76
71
dimensional crystals over the phospholipid bilayers that shield them antibodies on annexin A5 binding has been correlated with IgG anti-
from binding coagulation factors and it has been proposed that the bodies that recognize a specific epitope—domain I of β GPI in patients
72
2
protein may play a thrombomodulatory role on the surfaces of cells with APS who have thrombosis and spontaneous pregnancy losses.
52
lining the placental and systemic vasculatures. Annexin A5 is highly Figure 131–2 includes a model for this mechanism.
expressed on the apical membranes of placental syncytiotrophoblasts, Binding to Endothelial Surface Receptors Enhances Cell
the location where maternal blood interfaces with fetal cells. Preg- Signaling aPL antibodies can bind, injure, and activate cultured vascu-
73
nant annexin A5-null mice develop placental infarctions of fetuses and lar endothelial cells. 88–91 Cultured endothelial cells incubated with aPL
yield reduced litter sizes. Pregnant mice treated with anti–annexin A5 antibodies with specificity for cell surface β GPI express increased lev-
74
2
antibodies developed placental necrosis, fibrosis, and pregnancy loss. els of cell adhesion molecules triggered by their binding to cell surface
92
75
Dissociation of annexin A5 from the surface of human placental tro- β GPI. Annexin A2 serves as a receptor for β GPI, and anti-β GPI
93
62
2
2
2
phoblasts and human umbilical vein endothelial cells accelerates the antibodies may thereby stimulate expression of tissue factor on endothe-
coagulation of plasma exposed to those cells. Annexin A5 binds to the lial cells. In animal models, the signaling effects of aPL antibodies were
94
76
surfaces of endothelial cells and inhibits thrombin formation. 77 significantly reduced in mice treated with an anti-annexin A2 monoclo-
aPL antibody–antigen complexes disrupt the crystallization of nal antibody and also in annexin A2-null transgenic mice. These effects
95
annexin A5 and displace the protein from phospholipid membrane sur- of the aPL antibodies may be mediated by TLR-4 of the innate immu-
faces (Fig. 131–3). 78–81 In contrast to the LA phenomenon, aPL antibodies nity system, 96,97 although there are data indicating participation of other
Figure 131–3. Disruption of annexin A5 shield by
monoclonal antiphospholipid antibodies and β -gly-
2
coprotein I (β GPI). Atomic force microscopy picture
2
Annexin A5 shield showing the effect of a monoclonal aPL antibody
on a preformed annexin A5 crystal. The figure dem-
Exposed anionic onstrates the smooth lipid bilayer covered by the
phospholipids annexin A5 crystals, disrupted by antibody–β GPI
2
complexes (white circles) and exposing anionic phos-
Antibody/antigen pholipids (black holes) to coagulation factors and
complexes accelerated coagulation. (Modifed with permission from
Rand JH, Wu XX, Quinn AS, et al: Human monoclonal
antiphospholipid antibodies disrupt the annexin A5
anticoagulant crystal shield on phospholipid bilayers:
evidence from atomic force microscopy and functional
assay. Am J Pathol 163(3):1193–1200, 2003.)
Kaushansky_chapter 131_p2233-2252.indd 2236 9/18/15 5:10 PM