Page 488 - Williams Hematology ( PDFDrive )
P. 488
462 Part VI: The Erythrocyte Chapter 31: Structure and Composition of the Erythrocyte 463
Macrophages in erythroblastic islands not only affect erythroid the process of differentiation and is antecedent to a “precursor” cell,
differentiation and/or proliferation, but also perform other functions, the latter being identifiable by light microscopy by its morphologic
including rapid phagocytosis (<10 min) of extruded nuclei as a result characteristics (see Chap. 83, Fig. 83–2). In erythropoiesis, the ear-
of exposure of phosphatidylserine on the surface of the membrane sur- liest precursor is the proerythroblast. Erythroid progenitor cells are
22
rounding the nucleus. This phagocytosis is the reason for the inability identified as marrow cells capable of forming erythroid colonies in
to find extruded nuclei in marrow aspirates in spite of the fact that 2 semisolid medium in vitro under conditions in which the appropriate
million nuclei are extruded every second during steady-state erythro- growth factors are present. Progenitor cells also may be identified by
poiesis. A protective macrophage function linked to efficient phago- characteristic profiles of surface CD antigens using flow cytometry.
cytosis has been described. In normal mice, DNase II in macrophages Numerically, erythroid progenitors, BFU-E and CFU-E represent only
degrades the ingested nuclear DNA but in DNase II knockout mice the a minute proportion of human marrow cells. BFU-E range from
6
inability to degrade DNA results in macrophage toxicity with resultant 300 to 1700 × 10 mononuclear cells and CFU-E range from 1500 to
5
6
decrease in number of marrow macrophages and in conjunction with 5000 × 10 mononuclear cells. In vitro cultures using CD34+ cells
severe anemia. Macrophages can play both positive and negative regu- from blood, cord blood, and marrow as the starting material have
25
latory roles in human erythropoiesis but the mechanistic basis for these identified the critical cytokines required for erythroid differentiation
regulatory processes are not completely understood. 16,24 These processes and maturation and enabled the identification and isolation of pure
may play a role in the ineffective erythropoiesis in disorders such as cohorts of erythroid progenitors and erythroblasts at all stages of ter-
MDS, thalassemia, and malarial anemia. minal erythroid maturation. 4,5
Another potentially important role originally proposed for the
central macrophage is direct transfer of iron to developing erythroblasts Precursors
mediated by ferritin exchange between macrophages and erythroblasts Figure 31–2 shows the sequence of precursors as seen in marrow films.
13
(Chap. 42). Although this is an interesting concept, there is no defini- Figure 31–3 shows the marrow precursors as isolated by flow cytometry.
tive evidence for this exchange. Proerythroblasts On stained films, the proerythroblast appears as
a large cell, irregularly rounded or slightly oval. The nucleus occupies
13
approximately 80 percent of the cell area and contains fine chroma-
ERYTHROID PROGENITORS AND PRECURSORS tin delicately distributed in small clumps. One or several well-defined
nucleoli are present. The high concentration of polyribosomes gives
Early Progenitors the cytoplasm of these cells its characteristic intense basophilia. At
A “progenitor” in the hematopoietic system is defined as a marrow cell very high magnification, ferritin molecules are seen dispersed singly
that is a derivative of the pluripotent hematopoietic stem cell through throughout the cytoplasm and lining the clathrin-coated pits on the cell
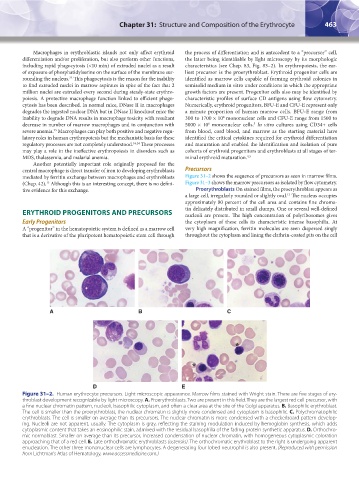
A B C
D E
Figure 31–2. Human erythrocyte precursors. Light microscopic appearance. Marrow films stained with Wright stain. There are five stages of ery-
throblast development recognizable by light microscopy. A. Proerythroblasts. Two are present in this field. They are the largest red cell precursor, with
a fine nuclear chromatin pattern, nucleoli, basophilic cytoplasm, and often a clear area at the site of the Golgi apparatus. B. Basophilic erythroblast.
The cell is smaller than the proerythroblast, the nuclear chromatin is slightly more condensed and cytoplasm is basophilic. C. Polychromatophilic
erythroblasts. The cell is smaller on average than its precursors. The nuclear chromatin is more condensed with a checkerboard pattern develop-
ing. Nucleoli are not apparent, usually. The cytoplasm is gray, reflecting the staining modulation induced by hemoglobin synthesis, which adds
cytoplasmic content that takes an eosinophilic stain, admixed with the residual basophilia of the fading protein synthetic apparatus. D. Orthochro-
mic normoblast. Smaller on average than its precursor, increased condensation of nuclear chromatin, with homogeneous cytoplasmic coloration
approaching that of a red cell. E. Late orthochromatic erythroblasts (asterisks). The orthochromatic erythroblast to the right is undergoing apparent
enucleation. The other three mononuclear cells are lymphocytes. A degenerating four-lobed neutrophil is also present. (Reproduced with permission
from Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
Kaushansky_chapter 31_p0459-0478.indd 463 9/18/15 10:58 PM