Page 644 - Williams Hematology ( PDFDrive )
P. 644
618 Part VI: The Erythrocyte Chapter 42: Iron Metabolism 619
the transit time, and mucus secretion all play roles in iron absorption. the intestinal villus cell by the divalent metal transporter (DMT)-1. 24,25
Red wine, contrary to popular belief, inhibits iron absorption, probably How iron transits within the enterocytes is not yet known. Basolateral
19
because of the presence of polyphenols. In mice, alcohol suppresses export of ferrous iron is mediated by ferroportin 26–28 in association with
30
20
the response of hepcidin to iron, and this may contribute to iron load- hephaestin and plasma ceruloplasmin to oxidize iron to the ferric
29
ing that is seen in some alcoholic subjects. state. Ferric iron is taken up by plasma apotransferrin. Figure 42–2 illus-
trates some of the steps that are thought to regulate iron transport across
IRON ABSORPTION the mucosal cell.
Iron normally enters the body through the gastrointestinal tract,
mostly through the enterocytes of the duodenum. The amount of iron IRON RECYCLING
absorbed is normally tightly regulated according to body needs. Active Role of the Monocyte–Macrophage System
erythropoiesis and/or iron deficiency increase absorption; iron over- In humans, the destruction and production of erythrocytes generates
load and systemic inflammation decrease absorption. Nevertheless, the most of the iron flux in and out of plasma (20 to 25 mg/day recycled in
amount of iron absorbed increases with the administered dose even adults compared to 1 to 2 mg/day absorbed). Iron from other cell types
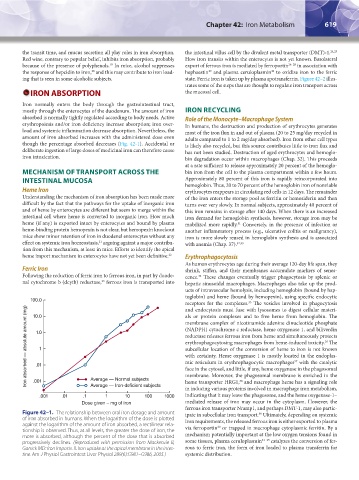
though the percentage absorbed decreases (Fig. 42-1). Accidental or is likely also recycled, but this source contributes little to iron flux and
deliberate ingestion of large doses of medicinal iron can therefore cause has not been studied. Destruction of aged erythrocytes and hemoglo-
iron intoxication. bin degradation occur within macrophages (Chap. 32). This proceeds
at a rate sufficient to release approximately 20 percent of the hemoglo-
MECHANISM OF TRANSPORT ACROSS THE bin iron from the cell to the plasma compartment within a few hours.
INTESTINAL MUCOSA Approximately 80 percent of this iron is rapidly reincorporated into
hemoglobin. Thus, 20 to 70 percent of the hemoglobin iron of nonviable
Heme Iron erythrocytes reappears in circulating red cells in 12 days. The remainder
Understanding the mechanism of iron absorption has been made more of the iron enters the storage pool as ferritin or hemosiderin and then
difficult by the fact that the pathways for the uptake of inorganic iron turns over very slowly. In normal subjects, approximately 40 percent of
and of heme by enterocytes are different but seem to merge within the this iron remains in storage after 140 days. When there is an increased
intestinal cell where heme is converted to inorganic iron. How much iron demand for hemoglobin synthesis, however, storage iron may be
heme (if any) is exported intact by enterocytes and bound by plasma mobilized more rapidly. Conversely, in the presence of infection or
31
heme-binding protein hemopexin is not clear, but hemopexin knockout another inflammatory process (e.g., ulcerative colitis or malignancy),
mice show minor retention of iron in duodenal enterocytes without any iron is more slowly reused in hemoglobin synthesis and is associated
effect on systemic iron homeostasis, arguing against a major contribu- with anemia (Chap. 37). 32,33
21
tion from this mechanism, at least in mice. Efforts to identify the apical
heme import mechanism in enterocytes have not yet been definitive. 22 Erythrophagocytosis
As human erythrocytes age during their average 120-day life span, they
Ferric Iron shrink, stiffen, and their membranes accumulate markers of senes-
Following the reduction of ferric iron to ferrous iron, in part by duode- cence. These changes eventually trigger phagocytosis by splenic or
34
23
nal cytochrome b (dcytb) reductase, ferrous iron is transported into hepatic sinusoidal macrophages. Macrophages also take up the prod-
ucts of intravascular hemolysis, including hemoglobin (bound by hap-
toglobin) and heme (bound by hemopexin), using specific endocytic
100.0
receptors for the complexes. The vesicles involved in phagocytosis
35
Iron absorbed — absolute amount (mg) .01 1 (NADPH) cytochrome c reductase, heme oxygenase 1, and biliverdin
and endocytosis must fuse with lysosomes to digest cellular materi-
als or protein complexes and to free heme from hemoglobin. The
10.0
membrane complex of nicotinamide adenine dinucleotide phosphate
1.0
reductase releases ferrous iron from heme and simultaneously protects
erythrophagocytosing macrophages from heme-induced toxicity. The
36
subcellular location of the conversion of heme to iron is not known
with certainty. Heme oxygenase 1 is mostly located in the endoplas-
mic reticulum in erythrophagocytic macrophages with the catalytic
37
face in the cytosol, and little, if any, heme oxygenase in the phagosomal
membrane. Moreover, the phagosomal membrane is enriched in the
Average — Normal subjects
heme transporter HRG1, and macrophage heme has a signaling role
.001
38
Average — Iron-deficient subjects
in inducing various proteins involved in macrophage iron metabolism,
.001 .01 .1 1 10 100 1000 indicating that it may leave the phagosome, and the heme oxygenase-1–
Dose given – mg of iron mediated release of iron may occur in the cytoplasm. However, the
ferrous iron transporter Nramp1, and perhaps DMT-1, may also partic-
Figure 42–1. The relationship between oral iron dosage and amount ipate in subcellular iron transport. Ultimately, depending on systemic
39
of iron absorbed in humans. When the logarithm of the dose is plotted iron requirements, the released ferrous iron is either exported to plasma
against the logarithm of the amount of iron absorbed, a rectilinear rela- via ferroportin or trapped in macrophage cytoplasmic ferritin. By a
40
tionship is observed. Thus, at all levels, the greater the dose of iron, the
more is absorbed, although the percent of the dose that is absorbed mechanism potentially important at the low oxygen tensions found in
progressively declines. (Reproduced with permission from Mackenzie B, some tissues, plasma ceruloplasmin 41–43 catalyzes the conversion of fer-
Garrick MD: Iron Imports. II. Iron uptake at the apical membrane in the intes- rous to ferric iron, the form of iron loaded to plasma transferrin for
tine. Am J Physiol Gastrointest Liver Physiol 289(6):G981–G986, 2005.) systemic distribution.
Kaushansky_chapter 42_p0617-0626.indd 619 9/17/15 6:26 PM